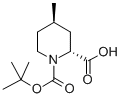
(+/-)-TRANS-N-BOC-4-METHYL-PIPECOLINIC ACID synthesis
- Product Name:(+/-)-TRANS-N-BOC-4-METHYL-PIPECOLINIC ACID
- CAS Number:123811-83-6
- Molecular formula:C12H21NO4
- Molecular Weight:243.3

124-38-9
129 suppliers
$214.00/14L
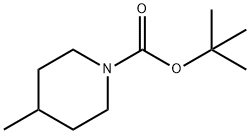
123387-50-8
65 suppliers
$65.00/500mg

123811-83-6
6 suppliers
inquiry
Yield:123811-83-6 89%
Reaction Conditions:
Stage #1: 4-methylpiperidine-1-carboxylic acid tert-butyl esterwith N,N,N,N,-tetramethylethylenediamine;sec.-butyllithium in diethyl ether;cyclohexane at -78 - 20; for 1 h;
Stage #2: carbon dioxide in diethyl ether;cyclohexane at -78; for 0.25 h;
Steps:
6 Preparation of compound 55
A solution of compound 54 (926.5 mg, 5.0 mmol) in Et2O (10.5 mL) was cooled to -78°C and treated with TMEDA (755 μL, 5.0 mmol) followed by slow addition of a 1.3 M cyclohexane solution of sec-butyllithium (4.6 mL, 6.0 mmol) over a 30 minute period. The reaction solution was then warmed to -20°C and maintained at that temperature for 30 minutes, after which the solution was re-cooled to -78°C and purged with gaseous carbon dioxide for 15 minutes. The reaction solution was then slowly warmed to 0°C and poured into a biphasic mixture of 1 N HCl (100 mL) and EtOAc (50 mL). The reaction solution was then extracted several times with EtOAc. The EtOAc extracts were combined, dried over Mg2SO4, filtered, and concentrated to provide compound 55 (1.07 g) in 89% yield as a colorless oil (a mixture of two cis enantiomers).
References:
EP2374454,2016,B1 Location in patent:Paragraph 0133; 0134
1003-67-4
234 suppliers
$26.00/25g

123811-83-6
6 suppliers
inquiry
1620-76-4
228 suppliers
$6.00/1g

123811-83-6
6 suppliers
inquiry
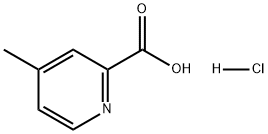
123811-73-4
4 suppliers
inquiry

123811-83-6
6 suppliers
inquiry
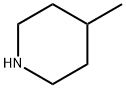
626-58-4
205 suppliers
$10.00/1g

123811-83-6
6 suppliers
inquiry