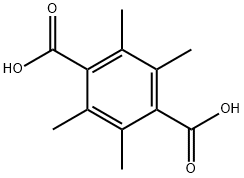
TETRAMETHYLTEREPHTHALIC ACID synthesis
- Product Name:TETRAMETHYLTEREPHTHALIC ACID
- CAS Number:14458-05-0
- Molecular formula:C12H14O4
- Molecular Weight:222.24

124-38-9
129 suppliers
$175.00/23402
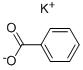
582-25-2
355 suppliers
$5.00/25g
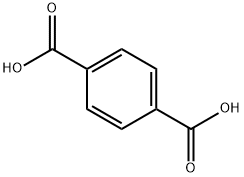
100-21-0
581 suppliers
$6.00/25g
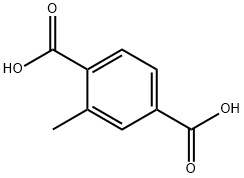
5156-01-4
111 suppliers
$32.00/250mg
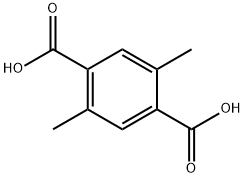
6051-66-7
112 suppliers
$45.00/500 mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

14458-05-0
52 suppliers
$123.00/250mg
Yield: 50%
Reaction Conditions:
with potassium acetate;potassium phthalate;zinc(II) oxide at 450; under 25858.1 Torr;Henkel Reaction;
Steps:
Potassium acetate, which melts (Condensed Chemical Dictionary, 6th edition) at 292°C, was used as a molten reaction medium for the Henkel reaction of potassium benzoate with and without ZnO as catalyst. It was thought that since the salt melts at a lower temperature than the carbonate eutectics, it might be possible to perform the reaction at a lower temperature. However, little Henkel reaction was observed at temperatures significantly below 400°C. Unexpectedly, however, on examining the reaction products of the black fused mass formed from the potassium acetate salt and the naphthoic or benzoate potassium salts at ca. 400°C and above, a significant portion of methylated diacids was observed. Thus, from a reaction conducted at 450°C and 500 psi of CO2 containing the following ingredients, 50g of potassium acetate, 5g of potassium phthalate, 1g of ZnO, a yield of about 50% of TPA and 20% of methylated TPA was unexpectedly observed. The methylated TPA was a mixture of 2-methyl 1,4 dicarboxy benzene, 2,5 dimethyl 1,4 dicarboxybenzene, 2,3,5 trimethyl 1,4 dicarboxy benzene, and 2,3,5,6 tetramethyl 1,4 dicarboxy benzene. The initial product appeared to be the 2-methyl species, however again surprisingly, the major components were the 2,5 dimethyl and the tetramethyl TPA (permethyl TPA). A possible explanation of this result is that the symmetrically di- or tetra- substituted salts are more stable or form particles which are more isolated from the reaction medium.
References:
Mossi & Ghisolfi International S.A. EP1232136, 2005, B1 Location in patent:Page/Page column 6-7
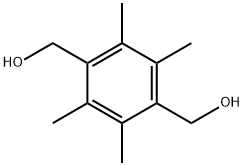
7522-62-5
55 suppliers
$60.00/100mg

14458-05-0
52 suppliers
$123.00/250mg
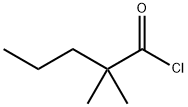
15721-22-9
27 suppliers
inquiry

7522-62-5
55 suppliers
$60.00/100mg

14458-05-0
52 suppliers
$123.00/250mg
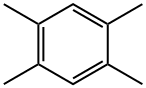
95-93-2
226 suppliers
$20.00/25g

14458-05-0
52 suppliers
$123.00/250mg
3022-16-0
34 suppliers
$33.39/250mg

14458-05-0
52 suppliers
$123.00/250mg