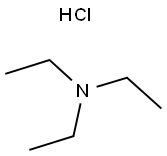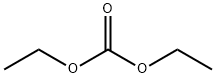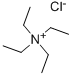
Tetraethylammonium Chloride synthesis
- Product Name:Tetraethylammonium Chloride
- CAS Number:56-34-8
- Molecular formula:C8H20ClN
- Molecular Weight:165.7
2603-10-3
63 suppliers
$17.67/1gm:

56-34-8
449 suppliers
$5.00/25g
Yield:-
Reaction Conditions:
with aniline in nitrobenzene
Steps:
6 EXAMPLE 6
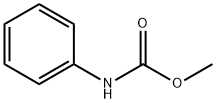
EXAMPLE 6 The procedure was the same as for Example 1 except that 0.23 g (1.40 millimole) tetraethylammoniumchloride was also provided to the reaction. Complete conversion of nitrobenzene occurred over 6.0 hours at 160° C. and yielded 0.077 mole methyl N-phenylcarbamate (77% selectivity based on nitrobenzene) and 0.071 mole aniline (21% selectivity to additional aniline based on nitrobenzene).
References:
Catalytica Associates;Haldor Topsoe US4705883, 1987, A
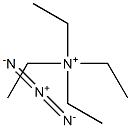
993-20-4
0 suppliers
inquiry
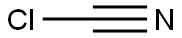
506-77-4
0 suppliers
inquiry
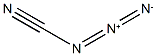
764-05-6
2 suppliers
inquiry

56-34-8
449 suppliers
$5.00/25g