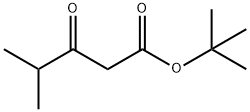
TERT-BUTYL 4-METHYL-3-OXOPENTANOATE synthesis
- Product Name:TERT-BUTYL 4-METHYL-3-OXOPENTANOATE
- CAS Number:94250-54-1
- Molecular formula:C10H18O3
- Molecular Weight:186.249
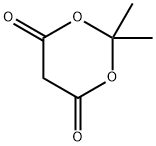
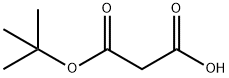
2033-24-1
666 suppliers
$6.00/25g
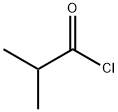
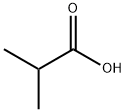
79-30-1
378 suppliers
$10.00/10g
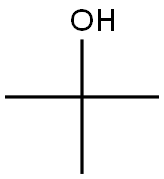
75-65-0
770 suppliers
$10.00/10ml

94250-54-1
10 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: cycl-isopropylidene malonate;isobutyryl chloridewith pyridine in dichloromethane at 0; for 2.5 h;
Stage #2: tert-butyl alcohol in toluene; for 6 h;Heating / reflux;
Steps:
25.1
1) To a solution of Meldrum's acid (14.41 g, 0.1 mol) and pyridine (16.2 mL, 0. 2 mol) in dichloromethane (100 mL) was added dropwise isobutyryl chloride (13.4 mL, 0.11 mol) at 0°C over 30 min. , and the mixture was stirred at 0°C for 2 hrs. The reaction mixture was poured into 0.5N hydrochloric acid, and the mixture was extracted with dichloromethane. The extract was washed with saturated brine and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure. A mixture of the obtained residue, tert- butanol (11.2 g, 150 mmol) and toluene (100 mL) was heated under reflux for 6 hrs. After allowing to cool to room temperature, the reaction mixture was washed with saturated brine and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure to give tert-butyl 4- methyl-3-oxopentanoate as a crude product (9.31 g). A mixture of the crude product (9.31 g), 25% aqueous ammonia (100 mL) and methanol (100 mL) was stirred at room temperature for 12 hrs. The reaction mixture was concentrated under reduced pressure, and partitioned between ethyl acetate and water. The organic layer was dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to give tert- butyl 3-amino-4-methylpent-2-enoate as a crude product (9.26 g).
References:
WO2005/42488,2005,A1 Location in patent:Page/Page column 108-109

2033-24-1
666 suppliers
$6.00/25g
513-36-0
232 suppliers
$28.00/25mL

94250-54-1
10 suppliers
inquiry
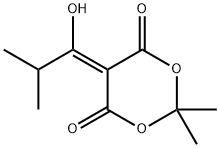
84794-38-7
12 suppliers
$110.00/500mg

75-65-0
770 suppliers
$10.00/10ml

94250-54-1
10 suppliers
inquiry