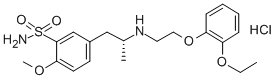
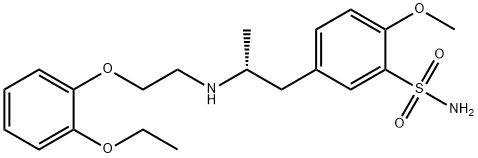
Tamsulosin hydrochloride synthesis
- Product Name:Tamsulosin hydrochloride
- CAS Number:106463-17-6
- Molecular formula:C20H29ClN2O5S
- Molecular Weight:444.97
![Benzenesulfonamide, 5-[2-[[2-(2-ethoxyphenoxy)ethyl]imino]propyl]-2-methoxy-](/CAS/20210111/GIF/852619-17-1.gif)
852619-17-1
0 suppliers
inquiry

106463-17-6
414 suppliers
$10.00/10mg
106133-20-4
217 suppliers
$49.00/50mg
Yield: 41.4 % ee
Reaction Conditions:
Stage #1:C20H26N2O5S;(S)-chloro[(1,2,3,4-η)-pentamethyl-2,4-cyclopentadien-1-yl] [N-(2-methoxy-3-dibenzofuranyl)-2-pyrrolidinecarboxamidato-κN1,κN2]iridium in acetonitrile at -3 - 3; for 0.5 h;
Stage #2: with formic acid;triethylamine in acetonitrile at -3 - 20;
Steps:
2.2
4.87 g of 2-methoxy-5-(2-oxopropyl)benzenesulfonamide, 3.62 g of 2-(2-ethoxyphenoxy)ethylamine and 10 mg of sodium acetate were added to 30 ml of acetonitrile. The mixture was heated while stirred under reflux for about 1 hour. Then, the solvent was evaporated off in vacuo, to give 7.84 g of an imine compound as a powder. The obtained imine compound was again dissolved in 70 ml of acetonitrile, and to this was added 1.0 g of anhydrous magnesium sulfate. The mixture was cooled to -3 to 3°C under argon atmosphere. To this mixture was added the whole amount of the above catalyst-containing mixture, and stirring continued at the same temperature for about 30 minutes. To this was added dropwise 12.0 ml of a mixed solution of formic acid/triethylamine (molar ratio: 5/2), and then stirring further continued at the same temperature for about 5 hours. After that, the reaction mixture was gradually returned to room temperature, and stirring continued overnight for completion of the reaction. After that, the solvent was evaporated off in vacuo and the residue was dissolved in methyl isobutyl ketone. Following this, extraction with aqueous methanesulfonic acid solution was performed. After the aqueous layer was rendered weakly alkaline with aqueous sodium hydroxide solution, separated oily material was extracted with methyl ethyl ketone. The extract solution was washed with saturated brine, dried and concentrated, to give 7.96 g of 5-[2-[(2-(2-ethoxyphenoxy)ethyl)amino]propyl]-2-methoxybenzenesulfonamide (crude crystal containing excess R-isomer) as a light brown powder. The obtained compound was analyzed using a chiral chromatography column (CHIRALPAK AD-H; manufactured by Daicel Chemical Industries, Ltd.) with a solvent made of n-hexane/2-propanol/diethylamine (800/200/1). The result showed that the optical purity of the R-isomer was 70.7%ee. Then, 6.0 g of the crude crystal was dissolved in 42.0 ml of aqueous acetone containing 10% water and to this was added 3.36 g of (R)-mandelic acid. The mixture was heat-dissolved, and then allowed to stand at 15 to 25°C overnight. The precipitate was collected by filtration, washed with acetone and dried, to give 4.56 g of (R)-tamuslosin (R)-mandelate, i.e., (R)-5-[2-[[2-(2-ethoxyphenoxy)ethyl]amino]propyl]-2-methoxybenzenesulfonamide (R)-mandelate as a colorless crystal. The optical purity of the R-isomer of the obtained compound was 97.0%ee. Moreover, recrystallization from aqueous acetone containing 10% water was performed to give 3.29 g of a purified product. The optical purity of the R-isomer of the purified product was 100%ee.Optical rotation: [α]D20 -31.90° (C = 0.5, MeOH) NMR: 1H-NMR (200MHz, DMSO-d6): δ 0.99-1.03 (3H, d), 1.23-1.30 (3H, t), 2.47-2.59 (1H, q), 2.97-3.05 (1H, q), 3.10-3.30 (1H, m), 3.14-3.20 (2H, t), 3.88 (3H, s), 3.94-4.04 (2H, q), 4.09-4.15 (2H, t), 4.43 (2H, m), 4.75 (1H, s), 6.82-7.43 (9H, m), 7.04 (2H, m), 7.58-7.60 (1H, d)
References:
Hamari Chemicals, Ltd. EP2172443, 2010, A1 Location in patent:Page/Page column 12