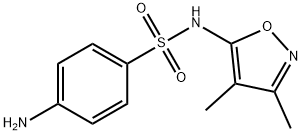
Sulfisoxazole synthesis
- Product Name:Sulfisoxazole
- CAS Number:127-69-5
- Molecular formula:C11H13N3O3S
- Molecular Weight:267.3
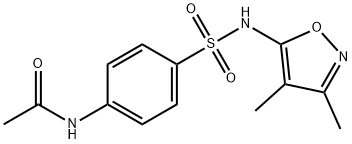
184644-22-2
2 suppliers
inquiry

127-69-5
256 suppliers
$5.00/100mg
Yield:127-69-5 69%
Reaction Conditions:
with acetic acid;zinc in acetonitrile at 0 - 20;
Steps:
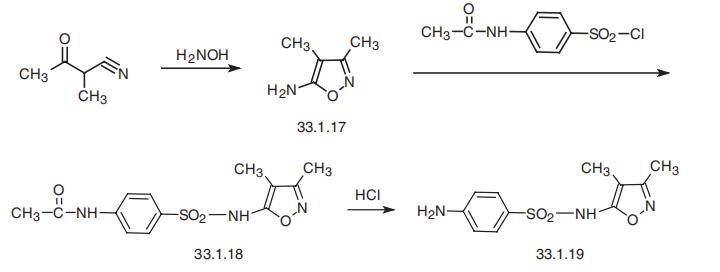
4.2.2. 4-Amino-N-(3,4-dimethylisoxazol-5-yl)benzenesulfonamide (14)
Nitro compound 13 (0.71 g, 2.40 mmol) was dissolved in a mixture of AcOH:CH3CN (20 mL, 1:1, v/v) and cooled to 0 °C. Zn powder (1.5 g) was added in small portions over a period of 10 min. After stirring the reaction for 3 h at rt, reaction mixture was filtered off and filtrate was evaporated to dryness. The resulting residue was purified by silica gel column chromatography (0-10% MeOH in EtOAc, v/v) to afford amine 14 (0.65 g, 69%) as a solid.
References:
Costanzi, Stefano;Santhosh Kumar;Balasubramanian, Ramachandran;Kendall Harden;Jacobson, Kenneth A. [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 17,p. 5254 - 5261]
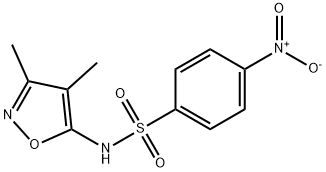
4206-74-0
2 suppliers
inquiry

127-69-5
256 suppliers
$5.00/100mg
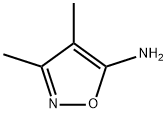
19947-75-2
133 suppliers
$14.14/250mgs:

127-69-5
256 suppliers
$5.00/100mg

19947-75-2
133 suppliers
$14.14/250mgs:
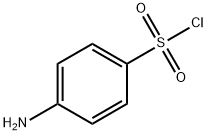
24939-24-0
38 suppliers
inquiry

127-69-5
256 suppliers
$5.00/100mg
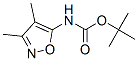
174079-07-3
3 suppliers
inquiry

127-69-5
256 suppliers
$5.00/100mg