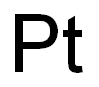
Platinum synthesis
- Product Name:Platinum
- CAS Number:7440-06-4
- Molecular formula:Pt
- Molecular Weight:195.08
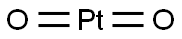
1314-15-4
417 suppliers
$60.57/200MG
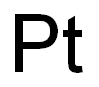
7440-06-4
375 suppliers
$19.00/250mg
Yield:-
Reaction Conditions:
with hydrogen in acetic acid; under 760.051 Torr; for 1.5 h;
Steps:
I.A EXAMPLE I
A) 10-Hydroxycamptothecin was prepared by subjecting camptothecin (3.2 g 0.0092 mol), 0.8 g of Pt0 (prepared by pre-reduction of 8 g of amorphous PtO2 in 80 ml of HOAc for 1.5 hr under 1 atmosphere hydrogen pressure) and acetic acid to 1 atm. of H2 for 8.5 h after which theoretical amount of H2 absorbed (slightly more than 0.4 l) and uptake of H2 gets slowed down The reaction mixture was degassed under steam of Helium and filtered through celite and washed with HOAc (20 ml). The resulting solution of 1, 2, 6, 7 tetrahydroxy-camptothecin was treated immediately with Pb (OAc)4 (6.4 g 0.014 mol) in portions and reaction mixture, stirred vigorously under Helium for 30 min. Gumy residue was obtained on evaporation of solvent which was triturated with cold water (100 ml) to produce light brown solid. The solid was collected, washed with cold water and air dried overnight when a mixture of 10-HCPT (44%), 10-AcHOCPT (26%) and unreacted CPT (32%) on HPLC basis was obtained. This crude mixture was combined with 150 ml of 50% HOAc and heated under reflux conditions overnight The reaction mixture was cooled, concentrated to 20 ml and treated with cold water (100 ml) to produce precipitate, which is filtered, washed with more cold water and dried to afford 2.1 g of solid containing HCPT (70%) AcCPT (1.2%) and CPT (21.3%) on the basis HPLC. Mixture was triturating with 0.5% aq HCl to dissolve the water-soluble. When insoluble CPT was removed by filtration. Water-soluble was extracted with chloroform and crystallized from boiling solution of 20% of MeOH in CHCl3 by adding EtOAC dropwise until turbidity appeared to obtain pure yellow HCPT which gives orange colored spot on TLC (CHCl3, acetone, MeOH 7:2:1), C20H16N2O5 (m/s 364), mp 268-270° C. UV. λmax. 222, 266, 330 and 382 IR (KBr) 3480 (OH), 1740 (Lactone) and 1655 (pyridone) cm-1; 1H NMR 0.88 (t, 3, C-18), 1.85 (m, 2, C-19 CH3), 5.35 (s, 2, C-17), 6.40 (s, C-20 OH), 7.22 (s, 1H, C-14), 7.28 (m, C-11 and C-12), 7.26 (d, 1, C-9), 8.38 (1, s, C-7), 10.3 (s, br, C-10 OH).
References:
US6660861,2003,B1 Location in patent:Page column 4, 5
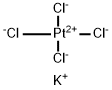
10025-99-7
454 suppliers
$19.00/250mg
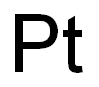
7440-06-4
375 suppliers
$19.00/250mg
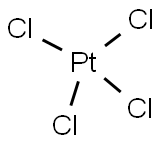
13454-96-1
243 suppliers
$17.00/100mg
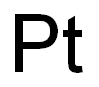
7440-06-4
375 suppliers
$19.00/250mg