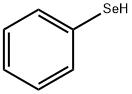
PHENYLSELENOL synthesis
- Product Name:PHENYLSELENOL
- CAS Number:645-96-5
- Molecular formula:C6H6Se
- Molecular Weight:157.07
108-90-7
671 suppliers
$10.00/25G

645-96-5
104 suppliers
$51.10/1g
Yield:645-96-5 68.4 g
Reaction Conditions:
Stage #1:chlorobenzene with magnesium in diethyl ether at 40; for 6 h;Inert atmosphere;
Stage #2: with selenium in diethyl ether at 0 - 10;Inert atmosphere;
Stage #3: with hydrogenchloride in diethyl ether;water; pH=< 1 at 0 - 30; for 1 h;Inert atmosphere;
Steps:
2; 3
Stirrer, it was charged with diethyl ether 600.2g magnesium four-necked flask of 3L capacity equipped with a thermometer and a reflux condenser tube 194.5g (8.00mol).The monochlorobenzene solution monochlorobenzene 900.2g of (8.00mol) was dissolved in diethyl ether 600.8g was prepared and added to 1 in an amount of 10 minutes of which the four-necked flask as described above.The reaction was heated to 40° C using an oil bath, to initiate the Grignard reaction.Then, was added dropwise the rest of monochlorobenzene solution is subjected to 6 hours, to obtain a phenyl magnesium chloride solution 2101.1g (pure content 1029.2g, 7.52mol). Stirrer, thermometer and phenyl magnesium chloride solution obtained in Production Example 2 in four-necked flask of 500mL capacity equipped with a reflux condenser 140.1g (pure content 68.4g, 0.50mol) was charged and the reaction mixture 0° C After cooling to , selenium powder 47.4g to (0.50mol) was added while keeping the range of 0 ~ 10° C , to obtain a reaction solution containing seleno magnesium halide.Stirrer, was charged thermometer and four-neck flask in 35 mass% hydrochloric acid 83.3g of 500mL volume equipped with a reflux condenser and (0.80mol) mixed solution of pure water 200.8g (less than pH1), to obtain after the entire amount of the reaction solution was added dropwise so as not to leave the range of 0 ~ 30° C were were incubated by continuing the stirring for about 1 hour, the organic layer was collected.Although analysis was performed by absolute calibration curve method by gas chromatography for the recovered organic layer, diphenyl diselenide is oxidized substance benzeneselenol was detected.Also, it was distilled off low-boiling components of the recovered organic layer and then acquires a colorless liquid 68.4g of benzeneselenol (0.44mol) by vacuum distillation.The yield was 85.8% with respect to the Grignard reagent.Note that the obtained liquid is benzeneselenol was determined from the boiling point is 79 (25mmHg).
References:
Sumitomo Seika Corporation;Takano, Kasumi;Shiraishi, Hiroyuki;Sakauey, Shigeki KR2015/60739, 2015, A Location in patent:Paragraph 0081-0085
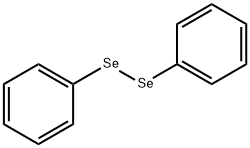
1666-13-3
241 suppliers
$10.00/1g

645-96-5
104 suppliers
$51.10/1g
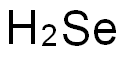
7782-49-2
272 suppliers
$27.00/25g

108-86-1
518 suppliers
$10.00/5g

645-96-5
104 suppliers
$51.10/1g

108-86-1
518 suppliers
$10.00/5g

645-96-5
104 suppliers
$51.10/1g