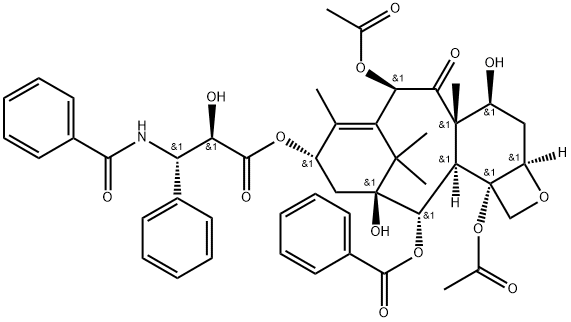
Paclitaxel synthesis
- Product Name:Paclitaxel
- CAS Number:33069-62-4
- Molecular formula:C47H51NO14
- Molecular Weight:853.92
Total Synthesis of Paclitaxel
The biosynthesis of paclitaxel involves the condensation of the three isoprenyl diphosphate (IPP) units with dimethylallyl diphosphate (DMAPP). Plants are unique in producing IPP and DMAPP by both the mevalonic pathway (MVA) in the cytosol or via the methylerythritol phosphate (MEP) pathway in the plastids.
Paclitaxel: biosynthesis, production and future prospects
1122-58-3
1104 suppliers
$6.00/1g

33069-62-4
994 suppliers
$28.00/5mg
Yield:33069-62-4 100%
Reaction Conditions:
with hydrogenchloride;water in ethanol at 0 - 20; for 2.41667 h;
Steps:
28
Example 28: Paclitaxel.; To a solution of 7-O-(triethylsilyl)pacl itaxel (7 mg) in ethanol (2 ml_) at 0 0C was added HCI (2 M, 2 ml_). The reaction was stirred for 2h and 25 minutes from 0 0C to room temperature. Next, EtOAc and aqueous sodium hydrogencarbonate were added at 0 0C. The mixture was extracted with dichloromethane, and the organic layer was dried over sodium sulfate. After filtration of the mixture and evaporation of the solvent, the crude product was purified by silica gel column chromatography to afford paclitaxel in quantitative yield as a white solid. 1H NMR (400 MHz, CDCI3): δ - 8.13 (d, J=8.4Hz, 2H), 7.74 (d, J=8.8Hz, 2H), 7.64-7.58 (m, 1 H), 7.53-7.46 (m, 5H), 7.44-7.34 (m, 5H), 6.98 (d, J=8.8Hz, 1 H), 6.27 (s, 1 H), 6.23 (t, J=8.8Hz, 1 H), 5.79 (dd, J=2.8Hz, J'= 6.4Hz, 1 H), 5.67 (d, J=6.8Hz, 1 H), 4.96 (d, J=8.0Hz, 1 H), 4.79 (d, J=2.4Hz, 1 H), 4.40 (dd, J=AAHz, J'=6.8Hz, 1 H), 4.30 (d, J=8.4Hz, 1 H), 4.20 (d, J=8.4Hz, 1 H), 3.80 (d, J=7.2Hz, 1 H), 3.54 (bs, 1 H), 2.59-2.50 (m, 1 H), 2.38 (s, 3H), 2.36-2.28 (m, 2H), 2.34 (s, 3H), 1.92-1.84 (m, 1 H), 1.80 (s, 3H), 1.68 (s, 3H), 1.25 (bs, 2H), 1.24 (s, 3H), 1.14 (s, 3H); 13C NMR (100 MHz, CDCI3): 203.6, 172.7, 171.3, 170.4, 167.0, 167.0, 142.0, 137.9, 133.7, 133.6, 133.2, 132.0, 130.2, 129.1 , 129.0, 129.0, 128.7, 128.7, 128.4, 127.0, 84.4, 81.2, 79.0, 76.5, 75.6, 74.9, 73.2, 72.2, 58.6, 55.0, 45.6, 43.2, 35.7, 35.6, 26.9, 22.6, 21.8, 20.8, 14.8, 9.5; HRMS (ESI): calcd for [M+Na] (C47H5I NOi4) requires m/z 876.3202, found 876.3191 : [α]D = -50.0 (c=0.1 , CHCI3); melting point: 195-197 0C.
References:
WO2010/62239,2010,A1 Location in patent:Page/Page column 21-22
![Benzenepropanoic acid, β-(benzoylamino)-α-[(triethylsilyl)oxy]-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4a,8,13,13-tetramethyl-5-oxo-4-[(triethylsilyl)oxy]-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (αR,βS)-](/CAS/20211123/GIF/135365-62-7.gif)
135365-62-7
5 suppliers
inquiry

33069-62-4
994 suppliers
$28.00/5mg
![Benzenepropanoic acid, β-(benzoylamino)-α-(2-ethoxyethoxy)-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4a,8,13,13-tetramethyl-5-oxo-4-[(triethylsilyl)oxy]-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (αR,βS)-](/CAS/20210305/GIF/439813-57-7.gif)
439813-57-7
1 suppliers
inquiry

33069-62-4
994 suppliers
$28.00/5mg

158722-23-7
20 suppliers
inquiry

33069-62-4
994 suppliers
$28.00/5mg
![Benzenepropanoic acid, β-(benzoylamino)-α-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-4-[(chloroacetyl)oxy]-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (αR,βS)-](/CAS/20210305/GIF/162081-12-1.gif)
162081-12-1
0 suppliers
inquiry

33069-62-4
994 suppliers
$28.00/5mg