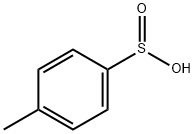
P-TOLUENESULFINIC ACID synthesis
- Product Name:P-TOLUENESULFINIC ACID
- CAS Number:536-57-2
- Molecular formula:C7H8O2S
- Molecular Weight:156.2
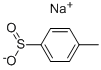
824-79-3
399 suppliers
$6.00/25g

536-57-2
147 suppliers
$34.00/1g
Yield:536-57-2 100%
Reaction Conditions:
with hydrogenchloride in tert-butyl methyl ether;water for 0.166667 h;
Steps:
25
Preparation 25; a- (p-Toluenesulfonyl) benzylisocyanide N-[Formyl] α-(p-toluenesulfonyl)benzylamine; Method A.; Add concentrated hydrochloric acid (3 mL) dropwise to a solution ofp-toluene- sulfinic acid sodium salt in water (20 mL) and test-butyl methyl ether (10 mL). Stir for 10 minutes and then separate the layers. Wash the organic layer with saturated aqueous sodium chloride, dry over sodium sulfate and concentrate under reduced pressure to provide 5 g ofp-toluenesulfinic acid. Combine this acid with benzaldehyde (4. 75 g, 44. 8 mmol), formamide (4. 9 g, 0. 11 mol), and camphorsulfonic acid (0. 86 g, 3. 7 mmol) and heat to 60°C for 18 hours. Remove the reaction from the heat and slurry the white solid in 3 : 1 hexanes : methanol. Filter the slurry to provide 7. 6 g (82%) of the desired product as a white solid. 1H-NMR (DMSO-d6) : § 9. 75 (d, 1H), 7. 98 (s, 1H), 7. 69 (d, 2H), 7. 53 (d, 2H), 7. 39 (m, 5H), 6. 36 (d, 1H), 2. 38 (s, 1H).; Method B.; Treat a solution ofp-toluenesulfinic acid sodium salt, (6. 0 g, 33. 7 mmol) in water (20 mL) and tert-butyl methyl ether (10 mL) dropwise with concentrated HCl (3 mL) and stir for 10 minutes. Separate the solution in a separator funnel and wash the organic layer with saturated aqueous sodium chloride. Dry the organic over Na2S04, filter, and remove the solvent to afford 5. 2 g (quantitative) ofp-toluenesulfinic acid. Combine the acid with benzaldehyde (2. 4 g, 22. 5 mmol), formamide (3. 8 g, 84. 2 mmol), and trimethylsilyl chloride (4. 0 g, 37. 0 mmol) in 30 mL of a 1 : 1 solution of toluene/acetonitrile. Heat the reaction to 50°C and stir for 5 hours. Cool the reaction and dilute with water (100 mL) and tert-butyl methyl ether (30 mL). Cool the solution in an ice bath and filter to afford 4. 5 g (70%) of desired product. Dry the solid under vacuum overnight to remove any residual water. 1H-NMR (DMSO) : 9. 75 (d, 1H), 7. 98 (s, 1H), 7. 69 (d, 2H), 7. 53 (d, 2H), 7. 39 (m, 5H), 6. 36 (d, 1H), 2. 38 (s, 1H).
References:
ELI LILLY AND COMPANY WO2005/80380, 2005, A1 Location in patent:Page/Page column 28-29
98-59-9
588 suppliers
$9.00/5g

536-57-2
147 suppliers
$34.00/1g

108-88-3
7 suppliers
$21.80/5 mL

536-57-2
147 suppliers
$34.00/1g
106-45-6
216 suppliers
$10.00/1g

536-57-2
147 suppliers
$34.00/1g
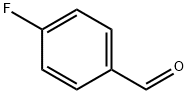
459-57-4
585 suppliers
$6.00/25g

536-57-2
147 suppliers
$34.00/1g
