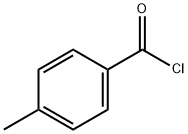
p-Toluoyl chloride synthesis
- Product Name:p-Toluoyl chloride
- CAS Number:874-60-2
- Molecular formula:C8H7ClO
- Molecular Weight:154.59
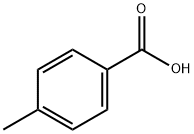
99-94-5
603 suppliers
$9.00/1g

874-60-2
422 suppliers
$10.00/5g
Yield:874-60-2 100%
Reaction Conditions:
with oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 35; for 1 h;
Steps:
Syntheses of Aryl Acid Chlorides (4a-f)
General procedure: The reaction scheme for the syntheses of aryl acid chlorides (4a-f) is shown in Scheme (1). Into a 250 mL two neck round bottomed flask equipped with a mechanical stirrer and a dropping funnel was placed in a thermostat bath. To a stirred solution of aryl acid (3a-f) 8 mM (3a: 0.98 g; 3b: 1.22g; 3c: 1.09 g; 3d: 1.34 g; 3e: 1.34 g; 3f: 1.18 g) in 100 mL CH2Cl2 at room temperature was added 16 mM (2.02 g) oxalyl chloride, 2 drops of DMF and the mixture was stirred at room temperature for 1h. The resulting mixture was concentrated under reduced pressure to afford acid chloride quantitatively and stored in air tight flask.
References:
Patel, Chirag Bhupendra;Dhaduk, Bhavin Babu;Parsania, Parsotam Hari [Letters in drug design and discovery,2015,vol. 12,# 6,p. 505 - 511]
13756-42-8
117 suppliers
$7.00/1g

874-60-2
422 suppliers
$10.00/5g

106-42-3
424 suppliers
$18.00/25ML

874-60-2
422 suppliers
$10.00/5g
100-20-9
337 suppliers
$14.00/25g
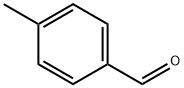
104-87-0
524 suppliers
$6.00/25g

874-60-2
422 suppliers
$10.00/5g
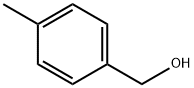
589-18-4
318 suppliers
$5.00/5g

874-60-2
422 suppliers
$10.00/5g