
Octadecanamine synthesis
- Product Name:Octadecanamine
- CAS Number:124-30-1
- Molecular formula:C18H39N
- Molecular Weight:269.51

638-65-3
60 suppliers
$54.00/5g

124-30-1
419 suppliers
$8.00/25g
Yield:124-30-1 98.2%
Reaction Conditions:
with sodium hydroxide;hydrogen;nickel in water at 135; under 14251.4 Torr; for 3 h;
Steps:
8
An autoclave was charged with 450 g of the product obtained in Example 1, 1.6 g of a Raney nickel catalyst as a hydrogenating catalyst, 0.9 g of 48% NaOH and 7.8 g of ion exchange water and the atmosphere in the vacant part of the autoclave was substituted with hydrogen to adjust the hydrogen pressure of the system to 1.9 MPa. Then, the mixture was raised at 135° C. and reacted for 3 hours. The resulting reaction product was subjected to compositional analysis using gas chromatography (gas chromatograph: HEWLETT PACKARD, column: Ultra-2 (inside diameter×length: 0.53 mm×15 m), manufactured by HEWLETT PACKARD) to measure the amount of stearylamine to be produced, to find that the yield was 98.2%.
References:
KAO CORPORATION US2005/59836, 2005, A1 Location in patent:Page/Page column 5
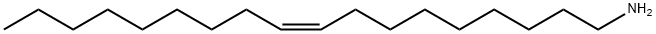
112-90-3
353 suppliers
$6.00/25g

124-30-1
419 suppliers
$8.00/25g
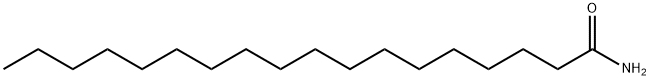
124-26-5
331 suppliers
$14.00/100g

124-30-1
419 suppliers
$8.00/25g
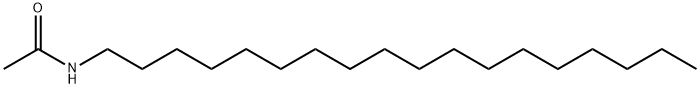
3906-22-7
5 suppliers
inquiry

124-30-1
419 suppliers
$8.00/25g

