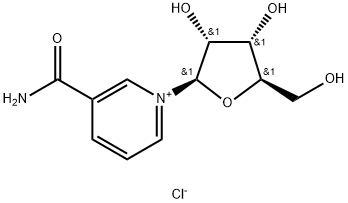
Nicotinamide riboside chloride synthesis
- Product Name:Nicotinamide riboside chloride
- CAS Number:23111-00-4
- Molecular formula:C11H15ClN2O5
- Molecular Weight:290.7
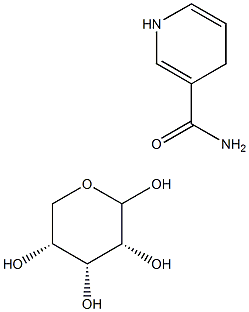
19132-12-8
30 suppliers
inquiry

23111-00-4
426 suppliers
inquiry
Yield:23111-00-4 100%
Reaction Conditions:
with ammonium chloride in water at 20; for 1 h;Solvent;
Steps:
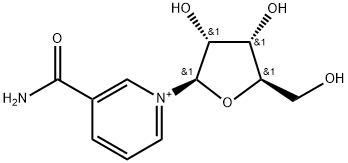
3A Example 3(A); Preparation of nicotinamide riboside, chloride salt (the 13-anomer form of which isshown in Figure 5).
A compound of formula (II), namely NRH (reduced nicotinamide riboside, shown in Figure 2; 50mg, 0.20mmol, leq), was dissolved in 5mL H20 and then ieq (i.e. 0.20mmol) of ammonium chloride was added in one portion. Activated charcoal (-10mg, i.e. 0.8Ommol) was then added and the mixturestirred at RT for -1 hr and then filtered and freeze-dried to give the chloride salt of nicotinamide riboside, quantitatively, i.e. 100% conversion and pure product.1H-NMR (D20, 400MHz) - ? 9.46 (s, 1H, aromatic), 9.12 (dt, 1H, J=6.3, 1.4 Hz, aromatic), 8.83 (dt, IH, J=8.2, 1.4 Hz, aromatic), 8.13 (dd, 1H, J=8.2, 6.3 Hz, aromatic>, 6.13 (d, 1H, J=4.3 Hz, H-i (anomeric)), 4.37 (t, 1H, J=4.7Hz, H-2), 4.31-4.34 (m, 1H, H-4), 4.21 (t, 1H, J=4.7Hz, H-3), 3.90(ABX, iH, Jaa13.0 Hz, Jab=3.5 Hz, H-5), 3.75 (ABX, 1H, Jaa=13.0 Hz, Jab=2.8 Hz, H-5).
References:
THE QUEEN'S UNIVERSITY OF BELFAST;MIGAUD, Marie;REDPATH, Philip;CROSSEY, Kerri;DOHERTY, Mark WO2015/14722, 2015, A1 Location in patent:Page/Page column 21
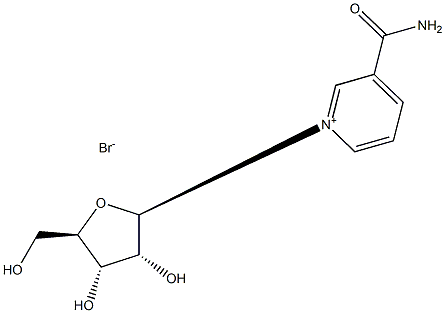
78687-39-5
6 suppliers
inquiry

23111-00-4
426 suppliers
inquiry
1341-23-7
344 suppliers
$28.00/1mg

23111-00-4
426 suppliers
inquiry
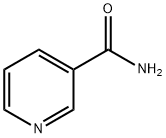
98-92-0
1232 suppliers
$5.00/5g

23111-00-4
426 suppliers
inquiry
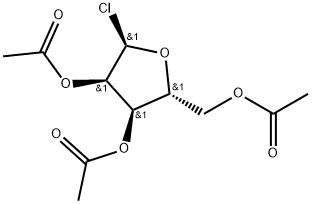
105499-44-3
1 suppliers
inquiry

23111-00-4
426 suppliers
inquiry
