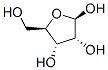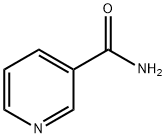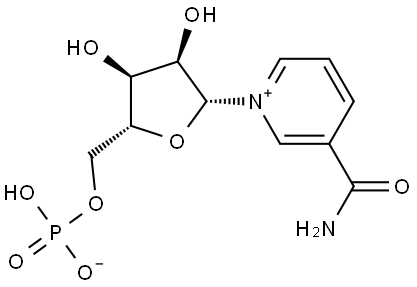
β-Nicotinamide Mononucleotide synthesis
- Product Name:β-Nicotinamide Mononucleotide
- CAS Number:1094-61-7
- Molecular formula:C11H15N2O8P
- Molecular Weight:334.22
Synthesis of β-nicotinamide mononucleotide (NMN)
A solution of NAD (3.5 g, 5.28 mmol) and ZrCl4(6.15 g, 26.4 mmol) in 500 ml water was stirred at 50°C for 30 min. The hydrolysis was monitored by TLC (SiO2EtOH/ 1 M NH4Ac [7 : 3]). The reaction was quenched with 245mL of a 0.5 M solution of Na3PO4. After adjusting to pH 7 with a 2 M solution of HCl, a white precipitate was formed. The suspension was centrifuged 8 min,1,000rpm, the supernatant was collected and the pellet was washed two times with 200 mL water. The combined supernatants wereconcentrated to 1/3 of its volume on a rotary evaporator. The remaining solution was purified with a column filled with Dowex 50WX8 (100-200 mesh, H+-Form, column-material: 2.5 x 30 cm). The column was loaded with 1.5 L5 % HCl and equilibrated with1.5L millipore water until pH 5 was reached. 100 mL of the concentrated solution was loaded on the ion exchange column and eluted with Milliporewater. The first cleavage product eluted was NMN (615 mg, 1.84 mmol,yield:35 %) and yielded a colorless solid after evaporation of the solvent, followed by AMP.
1H NMR (500MHz, D2O)δ: 9.48 (s, 1 H), 9.31 (d,J= 6.2 Hz, 1 H), 9.00 (d,J= 8.2 Hz, 1 H), 8.32 (dd,J= 8.2, 6.2 Hz, 1 H), 6.24 (d,J=5.4 Hz, 1 H), 4.68-4.64 (m, 1 H), 4.58 (t, 1 H), 4.48-4.45 (m, 1 H), 4.36–4.14 (m,J= 12.0, 2 H).
13C NMR (75 MHz, d2o) δ: 165.50, 145.65, 142.15, 139.53, 133.62, 128.19, 99.65, 87.18, 87.06, 77.42, 70.71, 63.89, 63.82.
31P NMR (202 MHz, D2O)δ:-0.03
23111-00-4
426 suppliers
inquiry

1094-61-7
830 suppliers
$7.00/100mg
Yield:1094-61-7 88.27%
Reaction Conditions:
with trimethyl phosphite;trichlorophosphate at 0; for 12 h;Inert atmosphere;
Steps:
1.4; 1.5; 2.4; 2.5; 3.4; 3.5
S4, adding 300 mL of trimethyl phosphate into the above reaction kettle, cooling to 0°C, adding 26.8 mL of phosphorus oxychloride under the protection of nitrogen, and reacting at a constant temperature for 12 hours;S5. Pour the reaction solution obtained in the above steps into rapidly stirring ice water, neutralize it with 6mol/L sodium hydroxide solution to pH=6, and transfer the solution to Dowexl*2 ionic resin, wash with water, and then use 5% CF3COOH was eluted, and the chromatographic solution was collected and dried to obtain β-nicotinamide mononucleotide with a molar yield of 88.27%.
References:
CN111647032,2020,A Location in patent:Paragraph 0027-0032
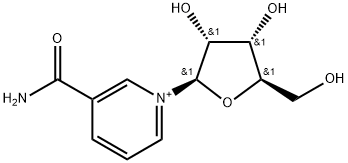
1341-23-7
344 suppliers
$31.00/1mg

1094-61-7
830 suppliers
$7.00/100mg
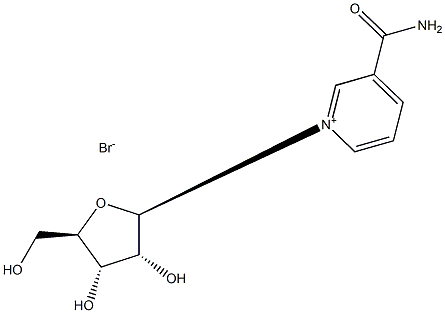
78687-39-5
6 suppliers
inquiry

1094-61-7
830 suppliers
$7.00/100mg
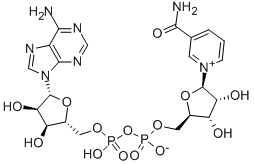
53-84-9
758 suppliers
$7.00/250mg

1094-61-7
830 suppliers
$7.00/100mg