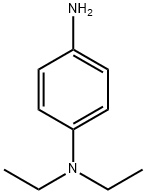
N,N-Diethyl-1,4-phenylenediamine synthesis
- Product Name:N,N-Diethyl-1,4-phenylenediamine
- CAS Number:93-05-0
- Molecular formula:C10H16N2
- Molecular Weight:164.25
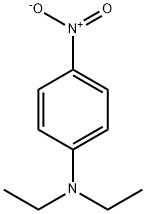
2216-15-1
6 suppliers
$20.00/1g

93-05-0
196 suppliers
$11.00/1g
Yield:93-05-0 90%
Reaction Conditions:
with palladium on activated charcoal;hydrogen in methanol at 20;
Steps:
4
4-Fluoronitrobenzene (316 mg, 2.2 mmol) was dissolved in DMSO (5 ml), potassium carbonate (464 mg, 3.4 mmol) and diethylamine (327 mg, 4.4 mmol) were added, and the mixture was stirred at 90° C. overnight.
Then, water was added to the reaction solution, and the mixture was extracted twice with ethyl acetate.
The organic layer was washed twice with saturated aqueous NaCl.
The organic layer was dried over Na2CO3, the solvent was evaporated, and the obtained residue was purified by silica gel chromatography (eluent; hexane:ethyl acetate (2:1)) to give compound Y181 (yield; 386 mg, 89%).
Compound Y181 (369 mg, 1.9 mmol) was dissolved in methanol (20 ml), Pd/C (141 mg) was added, and the mixture was stirred under a hydrogen atmosphere at room temperature overnight.
Then, the reaction solution was filtered through celite, the filtrate was concentrated under reduced pressure, and the obtained residue was purified by silica gel chromatography (eluent; hexane:ethyl acetate (1:1)) to give compound Y184 (yield; 280 mg, 90%).
Compound Y491 (mentioned later) (158 mg, 0.35 mmol) was dissolved in dichloromethane (5 ml), compound Y184 (143 mg, 0.9 mmol) and triethylamine (145 μl, 1.0 mmol) were added, and the mixture was stirred at room temperature for 1 hr.
Then, the solvent was concentrated under reduced pressure, and the obtained residue was purified by silica gel chromatography (eluent; chloroform:methanol (30:1)) to give the title compound (yield; 24 mg, 12%).
1H NMR (500 MHz, CDCl3) δ8.44 (s, 1H), 8.00 (d, 1H, J=8.0 Hz), 7.30 (d, 1H, J=8.0 Hz), 7.53 (t, 1H, J=8.0 Hz), 7.09 (bs, 1H), 6.87 (d, 2H, J=9.0 Hz), 6.49 (d, 2H, J=8.5 Hz), 5.45 (t, 1H, J=6.5 Hz), 4.06 (bs, 2H), 3.28 (dd, 4H, J=14.0, 7.0 Hz), 2.81-2.63 (m, 4H), 1.66-1.57 (m, 3H), 1.43 (s, 9H), 1.13 (t, 6H, J=7.0 Hz), 1.08-0.99 (m, 2H)
13C NMR (125 MHz, CDCl3) δ154.9, 147.1, 141.5, 141.1, 131.9, 131.0, 129.7, 126.9, 125.8, 122.8, 112.1, 79.6, 79.5, 77.4, 48.7, 44.5, 36.6, 29.6, 28.6, 12.6
HRMS (FAB-) m/z: [M-H]- calcd for C27H40N4O6S2, 579.2311. found, 579.2360
References:
KYOTO UNIVERSITY;Kakeya, Hideaki;Hattori, Akira;Takasu, Yasuaki;Fujii, Nobutaka;Oishi, Shinya;Kojima, Soichi;Hara, Mitsuko US2013/45977, 2013, A1 Location in patent:Paragraph 0219; 0220; 0221; 0222
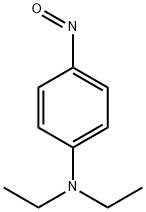
120-22-9
4 suppliers
$127.00/25g

93-05-0
196 suppliers
$11.00/1g
![Propanedioic acid, 2-[[[4-(diethylamino)phenyl]amino]methylene]-, 1,3-diethyl ester](/CAS/20210305/GIF/104007-17-2.gif)
104007-17-2
0 suppliers
inquiry

93-05-0
196 suppliers
$11.00/1g
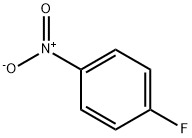
350-46-9
528 suppliers
$10.60/1gm:

93-05-0
196 suppliers
$11.00/1g
106-39-8
463 suppliers
$14.00/5g

93-05-0
196 suppliers
$11.00/1g