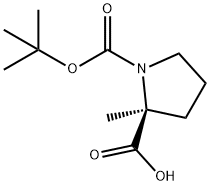
N-BOC-ALPHA-METHYL-L-PROLINE synthesis
- Product Name:N-BOC-ALPHA-METHYL-L-PROLINE
- CAS Number:103336-06-7
- Molecular formula:C11H19NO4
- Molecular Weight:229.27

24424-99-5
839 suppliers
$13.50/25G
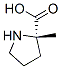
63399-73-5
6 suppliers
inquiry

103336-06-7
144 suppliers
$9.00/250mg
Yield:103336-06-7 64%
Reaction Conditions:
with triethylamine in 1,4-dioxane;water for 5 h;
Steps:
A.74.1 [0656] Step 1. 2S-Methyl-pyrrolidine-1,2-dicarboxylic Acid 1-tert-Butyl Ester
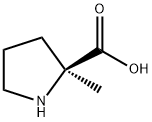
[0657] To a solution of 2S-methyl-pyrrolidine-2-carboxylic acid hydrobromide (1.50 g, 11.6 mmol; Bachem) in a mixture of H2O (15 ml) and dioxane (15 ml) was added Et3N (3.6 ml, 26 mmol) and di-tert-butyl dicarbonate (5.57 g, 25.5 mmol). The resultant solution stirred for 5 h, diluted with H2O (50 ml), washed with Et2O (50 ml), acidified to pH 2 with 10% HCl, and extracted with 10% MeOH/CHCl3 (2×100 ml). The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo to afford 1.1 g of white powder in 64% yield, which displayed an 1H NMR that matched previously reported (Khalil, et al., Tetrahedron Lett., 37, 3441-3444 (1996)) and was used without any further purification.
References:
Chu, Shao Song;Alegria, Larry Andrew;Bleckman, Ted Michael;Chong, Wesley Kwan Mung;Duvadie, Rohit K.;Li, Lin;Reich, Siegfried Heinz;Romines III, William Henry;Wallace, Michael B.;Yang, Yi US2003/225147, 2003, A1 Location in patent:Page 45

24424-99-5
839 suppliers
$13.50/25G
42856-71-3
205 suppliers
$19.00/250mg

103336-06-7
144 suppliers
$9.00/250mg
58632-95-4
267 suppliers
$7.00/5g
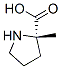
63399-73-5
6 suppliers
inquiry

103336-06-7
144 suppliers
$9.00/250mg
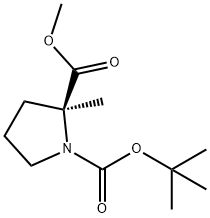
220060-17-3
17 suppliers
inquiry

103336-06-7
144 suppliers
$9.00/250mg

24424-99-5
839 suppliers
$13.50/25G

103336-06-7
144 suppliers
$9.00/250mg