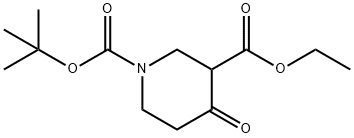
N-Boc-3-carboethoxy-4-piperidone synthesis
- Product Name:N-Boc-3-carboethoxy-4-piperidone
- CAS Number:98977-34-5
- Molecular formula:C13H21NO5
- Molecular Weight:271.31
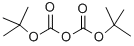
24424-99-5
863 suppliers
$13.50/25G
1454-53-1
249 suppliers
$5.00/5g

98977-34-5
203 suppliers
$5.00/250mg
Yield:98977-34-5 100%
Reaction Conditions:
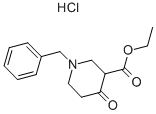
Stage #1: ethyl 1-benzyl-4-oxopiperidine-3-carboxylate hydrochloridewith hydrogen;palladium 10% on activated carbon in ethanol at 20; under 760.051 Torr; for 15 h;
Stage #2: di-tert-butyl dicarbonatewith triethylamine in ethanol at 20; for 16 h;
Steps:
1; 1.1
A solution of l-benzyl-3-carboethoxy-4-piperidone hydrochloride (IA) (10.0 g, 33.6 mmol) in ethanol (800 mL) and Pd/C (1.0 g, 10% w/w) was hydrogenated at 1 arm. for about 15 hours at room temperature. After the reaction was complete, triethylamine (19 mL, 134.3 mmol) and (Boc)2O (8.0 g, 36.9 mmol) were added to the mixture. The resulting solution was stirred at room temperature for 16 hours and the catalyst was removed by filtering through Celite. The filtrate was concentrated in vacuo, the resulting residue was dissolved in dichloromethane and extracted by washing with water. The organic phase was separated and dried over Na2SO4 and concentrated in vacuo to provide compound IB (9. Ig, 100%).
References:
WO2008/130584,2008,A1 Location in patent:Page/Page column 131; 135

24424-99-5
863 suppliers
$13.50/25G
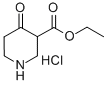
4644-61-5
169 suppliers
$10.00/1g

98977-34-5
203 suppliers
$5.00/250mg
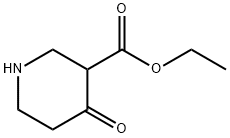
67848-59-3
54 suppliers
$110.00/50mg

24424-99-5
863 suppliers
$13.50/25G

98977-34-5
203 suppliers
$5.00/250mg

24424-99-5
863 suppliers
$13.50/25G
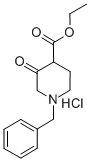
52763-21-0
249 suppliers
$6.00/1g

98977-34-5
203 suppliers
$5.00/250mg
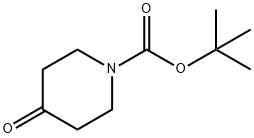
79099-07-3
0 suppliers
$5.00/5g
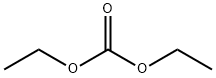
105-58-8
471 suppliers
$15.00/25g

98977-34-5
203 suppliers
$5.00/250mg