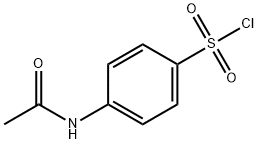
N-Acetylsulfanilyl chloride synthesis
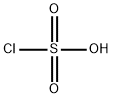
- Product Name:N-Acetylsulfanilyl chloride
- CAS Number:121-60-8
- Molecular formula:C8H8ClNO3S
- Molecular Weight:233.67
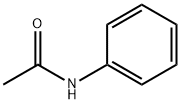
103-84-4
355 suppliers
$12.00/100g

121-60-8
346 suppliers
$10.00/1g
Yield:121-60-8 98.4%
Reaction Conditions:
Stage #1: N-phenylacetamidewith sulfonic chloride acid in 1,1,2,2-tetrachloroethylene at 10 - 50; for 1.33333 h;
Stage #2: with dichlorosulfoxide in 1,1,2,2-tetrachloroethylene at 63 - 70; for 1.33333 h;Temperature;
Steps:
1-3 Example 3
Molar ratio of acetanilide and chlorosulfonic acid is 1: 2.4; acetanilide tetrachlorethylene and a mass ratio of 1: 1.7; mass ratio of acetanilide and thionyl chloride is 1: 1.01; evaporated under reduced pressure tetrachlorethylene the amount of initially charged amount of 70% tetrachlorethylene. ASC preparation process: 1): In a 250 mL four-necked flask, add 23.4 g of tetrachloroethylene and 27.9 g (0.24 mol) of chlorosulfonic acid, stir and mix,Reduce the temperature to 11 °C and then slowly add 13.78 g of acetanilide (99% purity, 0.1 mol),Keep the addition temperature of acetanilide not more than 10 °C. After the addition of acetanilide, the temperature was raised to 45-50 °C and a chlorosulfonation reaction was performed for 80 minutes.2): The reaction mixture is then continuously heated up. When the temperature is 65-70 °C, 13.78 g of dichlorosulfoxide is added, the temperature is controlled at 63-68 °C and the reaction is performed for 80 minutes. 3): After the completion of the second reaction stage, 16.4 g of the solvent tetrachloroethylene was distilled off under reduced pressure. The reaction was continued at 63-68 °C for 50 min.Stop the reaction, lower the temperature to 20 °C and let it stand for 12h. The reaction product was isolated by dilution with an ice-water mixture.The mixture of the product ASC and water is subjected to suction filtration, and the obtained ASC product filter cake is dried in an oven at 50 °C. The ASC sample was analyzed by liquid chromatography. The purity of the ASC product was 98.5%, and the ASC yield was 98.4%.
References:
CN110590608,2019,A Location in patent:Paragraph 0031-0051
121-62-0
48 suppliers
$135.00/1g

121-60-8
346 suppliers
$10.00/1g
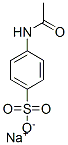
6034-54-4
4 suppliers
inquiry

121-60-8
346 suppliers
$10.00/1g
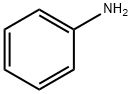
62-53-3
677 suppliers
$10.00/1g

121-60-8
346 suppliers
$10.00/1g