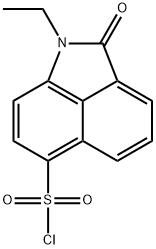
1-ETHYL-2-OXO-1,2-DIHYDROBENZO[CD]INDOLE-6-SULFONYL CHLORIDE synthesis
- Product Name:1-ETHYL-2-OXO-1,2-DIHYDROBENZO[CD]INDOLE-6-SULFONYL CHLORIDE
- CAS Number:32103-15-4
- Molecular formula:C13H10ClNO3S
- Molecular Weight:295.74
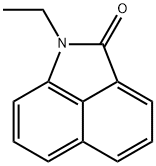
1830-56-4
25 suppliers
$155.00/50mg
![1-ETHYL-2-OXO-1,2-DIHYDROBENZO[CD]INDOLE-6-SULFONYL CHLORIDE](/StructureFile/ChemBookStructure5/GIF/CB7224298.gif)
32103-15-4
8 suppliers
$393.00/1g
Yield:32103-15-4 59%
Reaction Conditions:
with chlorosulfonic acid in chloroform at 0 - 50; for 6 h;
Steps:
4.3.4. 1-Ethyl-2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonylchloride (5)
To a solution of compound 4 (6.46 g, 33 mol) in chloroform (100 mL) was added chlorosulfonic (11.5 g, 99 mmol) dropwise at 0 °C for 10 min. The reaction mixture was heated at 50 °C for 6 h. The mixture was then poured into ice water. The reaction mixture was extracted with DCM (150 mL × 2). The organic layer was washed with brine and dried over Na2SO4. The solid was filtered off, and the filtrate was concentrated under reduced pressure. The resulting crude product was purified by silica gel chromatography with petroleum ether/ethyl acetate (5/1, v/v) to yield the title product as a yellow solid (5.75 g, 59 %). MS (ESI), m/z for C13H10ClNO3S ([M+H]+): Calcd 295.01, found 296.0.
References:
Zhang, Yan;Xue, Xiaoqian;Jin, Xiangyu;Song, Yu;Li, Jing;Luo, Xiaoyu;Song, Ming;Yan, Weiqun;Song, Hongrui;Xu, Yong [European Journal of Medicinal Chemistry,2014,vol. 78,p. 431 - 441]
![Benz[cd]indol-2(1H)-one](/CAS/GIF/130-00-7.gif)
130-00-7
229 suppliers
$5.00/5g
![1-ETHYL-2-OXO-1,2-DIHYDROBENZO[CD]INDOLE-6-SULFONYL CHLORIDE](/StructureFile/ChemBookStructure5/GIF/CB7224298.gif)
32103-15-4
8 suppliers
$393.00/1g
75358-95-1
1 suppliers
inquiry
![1-ETHYL-2-OXO-1,2-DIHYDROBENZO[CD]INDOLE-6-SULFONYL CHLORIDE](/StructureFile/ChemBookStructure5/GIF/CB7224298.gif)
32103-15-4
8 suppliers
$393.00/1g
134-32-7
374 suppliers
$9.00/1g
![1-ETHYL-2-OXO-1,2-DIHYDROBENZO[CD]INDOLE-6-SULFONYL CHLORIDE](/StructureFile/ChemBookStructure5/GIF/CB7224298.gif)
32103-15-4
8 suppliers
$393.00/1g
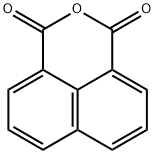
81-84-5
222 suppliers
$13.00/25g
![1-ETHYL-2-OXO-1,2-DIHYDROBENZO[CD]INDOLE-6-SULFONYL CHLORIDE](/StructureFile/ChemBookStructure5/GIF/CB7224298.gif)
32103-15-4
8 suppliers
$393.00/1g