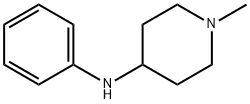
N-(1-METHYLPIPERIDIN-4-YL)ANILINE synthesis
- Product Name:N-(1-METHYLPIPERIDIN-4-YL)ANILINE
- CAS Number:22261-94-5
- Molecular formula:C12H18N2
- Molecular Weight:190.28

1445-73-4
461 suppliers
$15.00/25g
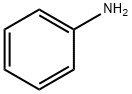
62-53-3
679 suppliers
$10.00/1g

22261-94-5
32 suppliers
$34.79/1g
Yield:22261-94-5 98%
Reaction Conditions:
Stage #1: 1-Methyl-4-piperidone;anilinewith sodium tris(acetoxy)borohydride;acetic acid in 1,2-dichloro-ethane at 0 - 20; for 16 h;
Stage #2: with sodium hydroxide;water in 1,2-dichloro-ethane;
Steps:
17
Example 17 1-Methyl-N-phenylpiperidin-4-amine A solution of aniline (2.0 g, 1.95 mL, 21.475 mmol), N-methyl-4-piperidone (2.64 mL, 21.475 mmol) and AcOH (1.21 mL, 21.475 mmol) in dry 1,2-dichloroethane (20 mL) was treated with NaBH(OAc)3 (6.82 g, 32.213 mmol) at 0° C. The reaction was brought to room temperature and stirred for over night (16 h). The reaction was basified with 1 N NaOH solution (40 mL) and product was extracted into CH2Cl2 (2*25 mL). The combined CH2Cl2 layer was washed with brine (15 mL) and dried (Na2SO4). Solvent was evaporated and crude was purified by column chromatography (2 M NH3 in MeOH:CH2Cl2, 5:95) to obtain compound 1-methyl-N-phenylpiperidin-4-amine (4.0 g, 98%) as a solid. 1H NMR (DMSO-d6) δ: 1.29-1.42 (m, 2H), 1.82-1.88 (m, 2H), 1.93-2.02 (m, 2H), 2.15 (s, 3H), 2.68-2.74 (m, 2H), 3.08-3.18 (m, 1H), 5.36 (d, 1H, J=8.1 Hz), 6.47 (t, 1H, J=7.2 Hz), 6.54 (d, 2H, J=7.8 Hz), 7.03 (t, 2H, J=7.2 Hz); ESI-MS (m/z, %) 191 (MH+, 100).
References:
US2008/234237,2008,A1 Location in patent:Page/Page column 49

1445-73-4
461 suppliers
$15.00/25g

62-53-3
679 suppliers
$10.00/1g
106-52-5
355 suppliers
$6.00/25g

22261-94-5
32 suppliers
$34.79/1g
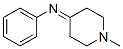
36796-46-0
2 suppliers
inquiry

22261-94-5
32 suppliers
$34.79/1g