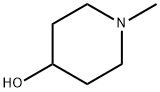
1-Methyl-4-piperidone synthesis
- Product Name:1-Methyl-4-piperidone
- CAS Number:1445-73-4
- Molecular formula:C6H11NO
- Molecular Weight:113.16
To a one-liter flask containing 350 ml of 20 % hydrochloric acid was added 86 g. of 1-methyl-3-carbethoxy-4-piperidone hyrdrochloride. After refluxing for one hour, the ferric chloride reagent gave no coloration. The solution was evaporated to dryness on a steam-bath at 10 mm. pressure. The solid product, heated at 100 °C for 4 hours at 0.1 mm and further dried over solid KOH for 24 hours, weighed 57.7 g, m.p. 80 - 120 °C.
Although this melting range goes above that of the pure comound 0.45 of crude material dissolved in 90 ml. of hot acetone gave 0.40 g of pure compoundd melting at 93 - 95 °C. Other samples of crude piperidone hydrochloride showed even higher melting points than the one mentioned above, yet this apperently impure material always gave good yields of sharp melting product when recrystallized.
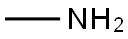
74-89-5
0 suppliers
$13.44/25ML

71-43-2
718 suppliers
$11.19/25ML

1445-73-4
461 suppliers
$15.00/25g
Yield:1445-73-4 91.7%
Reaction Conditions:
Stage #1: methylamine;benzenewith formaldehyd;toluene-4-sulfonic acid;diethyl 1,3-acetonedicarboxylateReflux;
Stage #2: with hydrogenchloride in water; for 4 h;Time;
Steps:
2; 3; 4; 5; 6 Example 6
A specific method for synthesizing N-methyl-4-piperidone includes the following steps:(1) Add diethyl 1,3-acetone dicarboxylate to benzene, place it in a reaction kettle, stir and mix well, add p-toluenesulfonic acid and mix wellAdd a catalyst, add formaldehyde and methylamine under stirring, and heat and reflux the reaction. After the reaction is completed, leave it to cool to room temperature and filter to remove the solid;The catalyst is the catalyst prepared in Example 1;(2) Add concentrated hydrochloric acid to the reaction kettle, stir and mix for 4h, then centrifuge,Take the hydrochloric acid layer and heat to reflux to decarboxylate. After the reaction is completed, adjust the pH to 12 with alkali.After cooling to room temperature, extraction was performed using dichloromethane.After drying over anhydrous sodium sulfate, dichloromethane was distilled off, and the N-methyl-4-piperidone was separated by chromatography.Diethyl 1,3-acetone dicarboxylate, formaldehyde,The molar ratio of methylamine used is 1: 3: 2; the mass ratio of diethyl 1,3-acetone dicarboxylate to the catalyst is 1: 1;The molar ratio of the amount of diethyl 1,3-acetone dicarboxylate to p-toluenesulfonic acid is 5: 3;The amount of diethyl 1,3-acetone dicarboxylate and benzene used is 1 mol / L;The concentration of concentrated hydrochloric acid is 12mol / L;The volume ratio of concentrated hydrochloric acid to benzene is 1.1: 2.The yield of the obtained N-methyl-4-piperidone was 91.7%, and the purity was 99.4%.
References:
CN110483374,2019,A Location in patent:Paragraph 0027-0056
106-52-5
355 suppliers
$6.00/25g

1445-73-4
461 suppliers
$15.00/25g
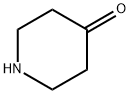
41661-47-6
0 suppliers
$45.00/250mg

74-88-4
352 suppliers
$15.00/10g

1445-73-4
461 suppliers
$15.00/25g
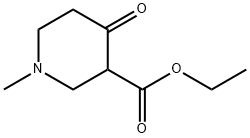
25012-72-0
33 suppliers
$65.00/50mg

1445-73-4
461 suppliers
$15.00/25g