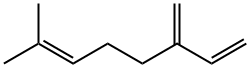
Myrcene synthesis
- Product Name:Myrcene
- CAS Number:123-35-3
- Molecular formula:C10H16
- Molecular Weight:136.23
![2H-Pyran, 2-[[(2E)-3,7-dimethyl-2,6-octadienyl]oxy]tetrahydro-](/CAS/20210305/GIF/59632-99-4.gif)
59632-99-4
0 suppliers
inquiry

123-35-3
423 suppliers
$27.00/25g
Yield:123-35-3 73.1%
Reaction Conditions:
with 18-crown-6 ether;potassium tert-butylate in tetrahydrofuran at 60; for 12 h;Inert atmosphere;
Steps:
2.2
Dissolve 1.40g (5.82mmol) of the intermediate in 5mL anhydrous tetrahydrofuran in a dry three-necked reaction flask, and dissolve 58.7mL (58.73mmol) 1M potassium tert-butoxide and 3.11g (11.77) in an organic solvent. mmol) 18-crown ether-6 was stirred and heated at 60°C under the protection of nitrogen to react for 14h. TLC (PE:EA=45:1) monitored the progress of the reaction. When the reaction was complete (no intermediate), the resulting reaction solution was stirred to room temperature , 100mL ether was added for extraction, and the organic phase was washed with 50mL water, 50mL saturated sodium bicarbonate aqueous solution and 50mL saturated brine successively, dried over anhydrous sodium sulfate, and purified by silica gel column chromatography (PE) to obtain 680mg (purity 99.81%) )13C2-Myrcene, the yield was 84.5%.
References:
CN111454114,2020,A Location in patent:Paragraph 0045; 0048
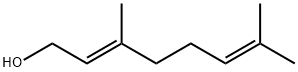
106-24-1
604 suppliers
$6.00/25g

123-35-3
423 suppliers
$27.00/25g

106-25-2
377 suppliers
$8.00/25g

123-35-3
423 suppliers
$27.00/25g

78-70-6
625 suppliers
$26.00/25mL

123-35-3
423 suppliers
$27.00/25g

106-24-1
604 suppliers
$6.00/25g

78-70-6
625 suppliers
$26.00/25mL

123-35-3
423 suppliers
$27.00/25g
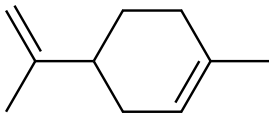
138-86-3
345 suppliers
$22.39/100ML