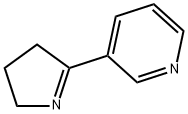
MYOSMINE synthesis
- Product Name:MYOSMINE
- CAS Number:532-12-7
- Molecular formula:C9H10N2
- Molecular Weight:146.19
Yield:532-12-7 77.2%
Reaction Conditions:
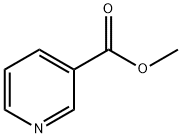
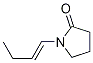
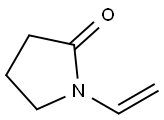
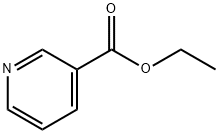
Stage #1:methyl 3-pyridinecarboxylate;1-(but-1-enyl) pyrrolidin-2-one with sodium hydride in N,N-dimethyl-formamide at 90; for 2 h;
Stage #2: with hydrogenchloride in water at 110; for 12 h;
Stage #3: with sodium hydroxide in water; pH=14 at 0;
Steps:
2
Sodium hydride (17.26 g, 072 mol of 60% dispersion in a mineral oil) was washed with toluene (25 ml x 2) to remove mineral oil and added to 25 ml of DMF. To this a solution containing 1-(but-1-enyl)-pyrrolidin-2-one (I, 50 g, 0.3597 mol) and methyl nicotinate (41.8 g, 0.3057 mol) in 50 ml of DMF was added. The reaction mixture was heated to 90°C for 2 hrs. DMF was partially removed under reduced pressure and 100 ml water and HCl (165 ml) were added. The reaction mixture was heated to 110°C for 12 hr, cooled and washed with ethyl acetate (50 ml x 2). The aqueous layer was cooled to 0°C, pH adjusted to about 14 using NaOH, extracted with dichloromethane (100 ml x 4), the extract dried over Na2SO4, the solvent removed completely and the crude solid was purified by high vacuum distillation to get myosmine (34.38 g, 77.2 % yield, 98.5% purity by GC).
References:
Divi's Laboratories Limited EP2487172, 2012, A1 Location in patent:Page/Page column 5
36740-10-0
13 suppliers
inquiry

532-12-7
226 suppliers
$35.00/50mg
123-75-1
547 suppliers
$10.00/5g
1120-90-7
307 suppliers
$5.00/250mg

110-86-1
857 suppliers
$9.00/5g

532-12-7
226 suppliers
$35.00/50mg

22083-74-5
104 suppliers
$82.00/100mg

532-12-7
226 suppliers
$35.00/50mg