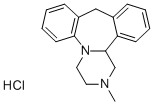
Mianserin hydrochloride synthesis
- Product Name:Mianserin hydrochloride
- CAS Number:21535-47-7
- Molecular formula:C18H21ClN2
- Molecular Weight:300.83
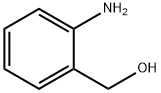
5344-90-1
339 suppliers
$6.00/5g
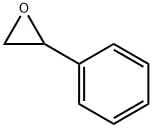
96-09-3
237 suppliers
$14.00/5g
50-00-0
895 suppliers
$10.00/25g

141-43-5
903 suppliers
$9.00/10g

21535-47-7
268 suppliers
$19.00/5mg
Yield:-
Reaction Conditions:
Stage #1:ethanolamine with benzaldehyde at 30 - 40; under 20 Torr; for 0.75 h;
Stage #2:styrene oxide at 90 - 140; for 2 h;
Stage #3:2-Aminobenzyl alcohol;formaldehydFurther stages;
Steps:
1.1; 1.2; 1.3; 1.4; 1.5; 1.6; 1.7; 1.8
(1) Injecting benzaldehyde into the reaction vessel and adding ethanolamine dropwise, the molar ratio of the two substances is 1:1.The reaction temperature is: 30-40 ° C,The reaction time is: 3 to 5 hours,After the reaction is complete,Add methanol and potassium borohydride for 3 to 5 hours.Filtration, collecting the filtrate, adding water to stir and standing, and distilling the oil layer under reduced pressure.The required vacuum is 0.096~0.1MPa,Receiving a fraction of 160-170 ° C,Intermediate I;(2), the intermediate I was placed in the reaction vessel, and the styrene oxide was added dropwise at a temperature of 90 to 100 ° C, and the molar ratio of the two materials was 1:1, and the reaction was carried out at 120 to 140 ° C for 2 hours. Intermediate II;(3) In step 2), pyridine and chlorobenzene are charged, and thionyl chloride is added dropwise at 50 to 60 ° C, and the molar ratio of thionyl chloride to styrene oxide in step 2) is 2.2:1.The reaction temperature is: 60 to 70 ° C,The reaction time is: 2 to 3 hours,Adding sodium hydroxide to adjust pH 5~6 to obtain intermediate III;(4), in step 3), put potassium carbonate, water, chlorobenzene, o-aminobenzyl alcohol,The molar ratio of o-aminobenzyl alcohol to styrene oxide in step 2) is 0.8:1,The reaction is refluxed for 4 to 6 hours, and the solvent is distilled off under reduced pressure in a water-repellent layer.Fumaric acid,Acetone is put into the reaction vessel for reflux for 0.5 to 1 hour.Cooling filtration,Drying intermediate IV;(5), the concentrated sulfuric acid is added to the reaction kettle, and the molar ratio of concentrated sulfuric acid to the styrene oxide in the step 2) is 12.3:1.Temperature control 30 ° C ~ 40 ° C,Add intermediate IV,Insulation reaction for 30 to 50 minutes,Then, the temperature is raised at 70 to 80 ° C for 1 hour.Put ice water into the reaction kettle to cool down,Stir at 34 to 36 ° C for 1 hour.Cooling to filter solids, then toluene, ethanol, sodium carbonate is put into the reaction kettle,The molar ratio of sodium carbonate to concentrated sulfuric acid is 12.3:4, and the reaction is carried out at 75 ° C for 40 to 60 minutes.Layering, washing to neutral, distilling off the solvent under reduced pressure to give intermediate V;(6), input toluene in the reaction tank,Add intermediate V,Add ethyl chloroformate dropwise,The molar ratio of ethyl chloroformate to styrene oxide in step 2) is 1.42:1,Reflux for 3 to 4 hours,Evaporate the solvent under reduced pressure.Then n-butanol,Potassium hydroxide is put into the reaction kettle,The molar ratio of potassium hydroxide to styrene oxide in step 2) is 3.1:1.Reflux for 3 to 4 hours,Add water and stir to layer,Wash with saturated saline until neutral,Go to the saline layer,Intermediate VI;(7), put formaldehyde into the reaction kettle,Add intermediate VI and reflux for 1 hour.The molar ratio of the formic acid, formaldehyde and formic acid to the styrene oxide in the step 2) is 4.5:12:1.After the completion of the dropwise addition, the mixture was refluxed for 6 hours, and the solvent was evaporated under reduced pressure, and the temperature was lowered to 30 to 40 ° C.Adding sodium hydroxide, toluene, water, stirring and layering, and distilling off the solvent under reduced pressure to obtain intermediate VII;(8), put acetone in the reaction vessel, add intermediate VII, heat to dissolve, adjust pH 2 to 3 with hydrochloric acid - ethyl acetate, coldHowever, it is filtered, dried and refined.
References:
Renhetang Pharmaceutical Co., Ltd.;Lin Xiangyu;Yao Qisheng;Nie Changsheng CN108250203, 2018, A Location in patent:Paragraph 0009