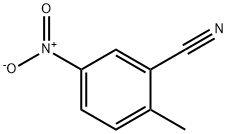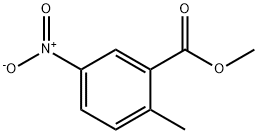
Methyl 5-nitro-2-methylbenzoate synthesis
- Product Name:Methyl 5-nitro-2-methylbenzoate
- CAS Number:77324-87-9
- Molecular formula:C9H9NO4
- Molecular Weight:195.17

67-56-1
776 suppliers
$7.29/5ml-f
201230-82-2
1 suppliers
inquiry
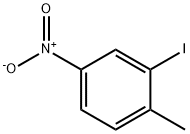
7745-92-8
152 suppliers
$9.00/1g

77324-87-9
208 suppliers
$6.00/1g
Yield: 37%
Reaction Conditions:
with triethylamine;diphenylphosphinopropane;palladium diacetate in methanol at 110; under 3000.3 Torr; for 18 h;
Steps:
104.a EXAMPLE 104; (E)-3-Ethyl-7-[3-(3-hydroxymethyl-4-methylphenoxymethyl)phenyl]non-6-en-3-ol; [01155] a) Methyl 2-Methyl-5-nitrobenzoate
23.2 g (88 mmol) of 2-iodo-4-nitrotoluene are dissolved in 400 ml of anhydrous methanol and then placed in a steel reactor. 25 ml (177 mmol) of triethylamine, 1.97 g (8.8 mmol) of palladium diacetate and 7.3 g of diphenyphosphinopropane are added and the reaction medium is subjected to a carbon monoxide pressure of 4 bar and heated at 110° C. for 18 hours. The medium is then brought to ambient pressure and temperature, dissolved in dichloromethane and filtered on celite. After evaporation, the residue is purified by chromatography on a silica column. An orange-coloured powder is obtained (m=6.3 g, Y=37%).
References:
Galderma Research & Development S.N.C. US6689922, 2004, B1 Location in patent:Page column 86
1975-52-6
284 suppliers
$12.00/1 g

74-88-4
353 suppliers
$15.00/10g

77324-87-9
208 suppliers
$6.00/1g

67-56-1
776 suppliers
$7.29/5ml-f

1975-52-6
284 suppliers
$12.00/1 g

77324-87-9
208 suppliers
$6.00/1g

1975-52-6
284 suppliers
$12.00/1 g

77324-87-9
208 suppliers
$6.00/1g