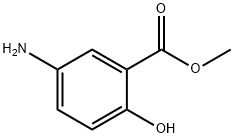
METHYL 5-AMINOSALICYLATE synthesis
- Product Name:METHYL 5-AMINOSALICYLATE
- CAS Number:42753-75-3
- Molecular formula:C8H9NO3
- Molecular Weight:167.16
Yield: 62%
Reaction Conditions:
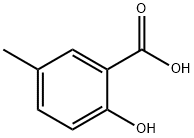
Stage #1:methanol;5-Methylsalicylic acid with sulfuric acidReflux;
Stage #2: with sodium hydroxide in methanol;dichloromethane;water; pH=7
Steps:
5-Amino-2-hydroxy-benzoic acid methyl ester 19; 5-Methyl salicylic acid (10 g, 65.3 mmol, 1 eq) was dissolved in methanol (80 ml) and conc. H2SO4 (10 ml) was added carefully. The mixture was heated to reflux temperature overnight and was then allowed to cool to ambient temperature and was then poured into a separating funnel and water (100 ml) and DCM (100 ml) were added. The pH was adjusted to 7 with dil. aq. NaOH and the organic layer was poured off. The aqueous layer was then extracted with DCM (2×50 ml) and the combined organic layers were washed with water (2×100 ml), were washed with brine (50 ml), were dried over Na2SO4, filtered and the solvent was removed in vacuo. This gave 9.778 g (62%) of an off white solid. 1H-NMR (DMSO D6) 500 MHz: δ (ppm)=3.85 (3H, s, CH3), 4.78 (2H, br.s, NH2), 6.70 (1H, d, J=8.7 Hz, CCHCHCN), 6.82 (1H, dd, J=2.9 Hz, J=8.7 Hz, CCHCHCN), 7.01 (1H, d, J=2.9 Hz, CCHCN), 9.74 (1H, s, COH). 13CNMR (DMSO D6) 125 MHz: δ (ppm)=52.1 (CH3), 112.1 (C), 112.8 (CH), 117.5 (CH), 123.0 (CH), 141.0 (C), 151.5 (C), 169.6 (CO). IR Spectrum; evaporated film: v(cm-1)=3408, 3328, 3220, 3082, 2958, 1675, 1616, 1485, 1441, 1303, 1231, 1083. MS-ES (positive): 168.06 (M+H+).
References:
Desreumaux, Pierre;Bellinvia, Salvatore;Chavatte, Philippe;Baroni, Sergio US2011/39808, 2011, A1 Location in patent:Page/Page column 50
17302-46-4
153 suppliers
$6.00/1g

42753-75-3
90 suppliers
$30.00/1g

67-56-1
757 suppliers
$7.29/5ml-f
89-57-6
781 suppliers
$5.00/50mg

42753-75-3
90 suppliers
$30.00/1g
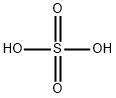
7664-93-9
6 suppliers
$21.64/1003

89-57-6
781 suppliers
$5.00/50mg

42753-75-3
90 suppliers
$30.00/1g

89-57-6
781 suppliers
$5.00/50mg

42753-75-3
90 suppliers
$30.00/1g