Methyl 4,5-dimethoxy-3-hydroxybenzoate synthesis
- Product Name:Methyl 4,5-dimethoxy-3-hydroxybenzoate
- CAS Number:83011-43-2
- Molecular formula:C10H12O5
- Molecular Weight:212.2

67-56-1
757 suppliers
$7.29/5ml-f
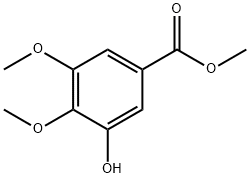
1916-08-1
76 suppliers
$89.29/250mg

83011-43-2
130 suppliers
$50.00/250mg
Yield:83011-43-2 97%
Reaction Conditions:
with sulfuric acid at 20; for 24 h;
Steps:
Methyl 3-hydroxy-4,5-dimethoxybenzoate (70) (Scheme 6).
To a solution of acid 69 (3.0 g, 151.4 mmol) in methanol (30 mL) was added sulfuric acid (0.5 mL). The solution was stirred at room temperature for 24 h, after which time the solvent was removed in vacuo. The crude residue was partitioned between EtOAc and water. The aqueous layer was extracted twice with EtOAc, and the combined organic extracts were washed with water then brine, dried over MgSO4, and concentrated in vacuo. The crude product was purified by column chromatography (EtOAc/n-heptane) to give pure compound 70 (3.13 g, 97%) as an off-white solid; mp (EtOAc/n- heptane) 68 °C (lit. 72-74 °C);49 1H NMR (CDCl3, 400 MHz) δ (ppm) 3.89 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.82 (s, 1H, ArOH), 7.20 (d, J = 2.0 Hz, 1H, ArH), 7.31 (d, J = 2.0 Hz, 1H, ArH). IR ν cm-1: 756, 995, 1006, 1110, 1162, 1200, 1270, 1356, 1594, 1704, 3403.
References:
Ghinet, Alina;Rigo, Beno?t;Hénichart, Jean-Pierre;Le Broc-Ryckewaert, Delphine;Pommery, Jean;Pommery, Nicole;Thuru, Xavier;Quesnel, Bruno;Gautret, Philippe [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 20,p. 6042 - 6054] Location in patent:supporting information; experimental part
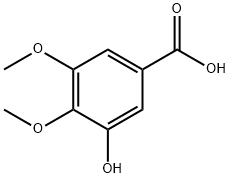
149-91-7
705 suppliers
$5.00/25g

74-88-4
349 suppliers
$15.00/10g
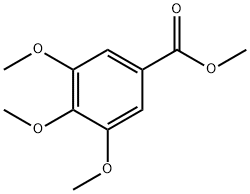
1916-07-0
437 suppliers
$6.00/25g
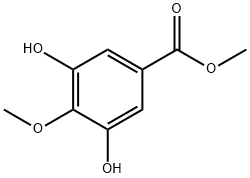
24093-81-0
51 suppliers
$45.00/10mg

83011-43-2
130 suppliers
$50.00/250mg
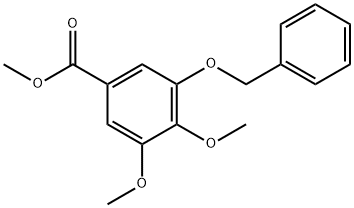
26409-24-5
7 suppliers
$57.00/1ea

83011-43-2
130 suppliers
$50.00/250mg

1916-08-1
76 suppliers
$89.29/250mg

83011-43-2
130 suppliers
$50.00/250mg
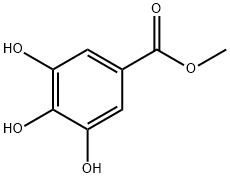
99-24-1
430 suppliers
$10.00/5g

74-88-4
349 suppliers
$15.00/10g

24093-81-0
51 suppliers
$45.00/10mg

83011-43-2
130 suppliers
$50.00/250mg