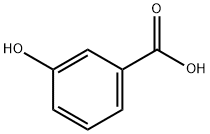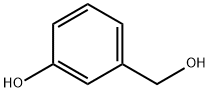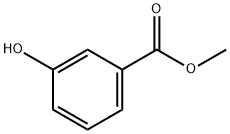
Methyl 3-hydroxybenzoate synthesis
- Product Name:Methyl 3-hydroxybenzoate
- CAS Number:19438-10-9
- Molecular formula:C8H8O3
- Molecular Weight:152.15

67-56-1
776 suppliers
$7.29/5ml-f
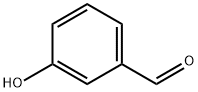
100-83-4
648 suppliers
$5.00/10g

19438-10-9
258 suppliers
$6.00/5g
Yield:19438-10-9 98%
Reaction Conditions:
with palladium 10% on activated carbon;oxygen;sodium carbonate at 100; under 15001.5 Torr; for 1 h;Microwave irradiation;Green chemistry;
Steps:
General procedure for aerobic aldehyde and alcohol esterification
General procedure: Na2CO3 (2 equiv) was dissolved in MeOH (1 mL) and sonicated with a US bath for 10 sec (20.3 kHz, 60 W). The substrate (aldehyde or alcohol, 1 mmol) and 10% Pd/C (5% Pd/mol of substrate) were added to this mixture. The reaction was carried out under magnetic stirring in a MW reactor Synth-Wave. The 1 L pressure-resistant PTFE cavity (up to 200 bar) equipped with a 15 position vial rack was loaded with O2 (2.5 bar) followed by the addition of N2 up to 20 bar total pressure. The reaction was irradiated for an appropriate reaction temperature ranging from 90 to 120 °C (average power 300 W), and for 1 to 2 hours (see Table 2 and Table 3). The mixture was then filtered off through celite, the catalyst washed with MeOH and the solvent evaporated under vacuum. Isolated yields for all substrates reported were obtained using these conditions.
References:
Caporaso, Marina;Cravotto, Giancarlo;Georgakopoulos, Spyros;Heropoulos, George;Martina, Katia;Tagliapietra, Silvia [Beilstein Journal of Organic Chemistry,2014,vol. 10,p. 1454 - 1461] Location in patent:supporting information
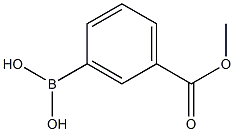
99769-19-4
274 suppliers
$10.00/1g

19438-10-9
258 suppliers
$6.00/5g
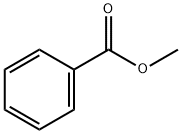
93-58-3
506 suppliers
$13.50/100 mL

19438-10-9
258 suppliers
$6.00/5g
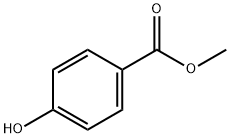
99-76-3
785 suppliers
$5.00/25g
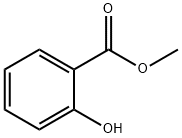
119-36-8
1022 suppliers
$5.00/10g