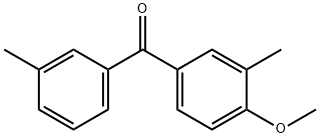
METHOXYPHENONE synthesis
- Product Name:METHOXYPHENONE
- CAS Number:41295-28-7
- Molecular formula:C16H16O2
- Molecular Weight:240.3
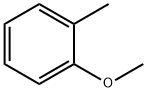
578-58-5
271 suppliers
$15.00/5 g
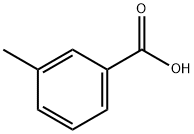
99-04-7
467 suppliers
$8.00/100g

41295-28-7
64 suppliers
$55.00/10mg
Yield:41295-28-7 60%
Reaction Conditions:
in PPA
Steps:
2 Preparation of 4-methoxy-3,3'-dimethylbenzophenone
EXAMPLE 2 Preparation of 4-methoxy-3,3'-dimethylbenzophenone m-Toluic acid (13.6 g, 0.10 mole) and o-methylanisole (12.2 g, 0.10 mole) are combined in 50 ml of polyphosphoric acid (PPA) and warmed with stirring to 80°C for 2 hours. The cooled reaction mixture is then poured into ice water and extracted into ethyl acetate (200 ml) and washed with 5% sodium hydroxide to give 14.4 g of product (60%), m.p. 62°-4°C, upon removal of the solvent in vacuo.
References:
Rohm and Haas Company US3954875, 1976, A

578-58-5
271 suppliers
$15.00/5 g
1711-06-4
327 suppliers
$6.00/25g

41295-28-7
64 suppliers
$55.00/10mg