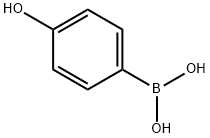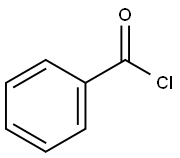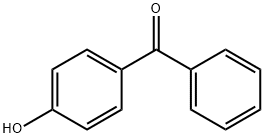
4-Hydroxybenzophenone synthesis
- Product Name:4-Hydroxybenzophenone
- CAS Number:1137-42-4
- Molecular formula:C13H10O2
- Molecular Weight:198.22
? with phenol in the presence of aluminium chloride in carbon disulfide at 0° (90%) or in the presence of zinc oxide in a water bath;
? with p-bromophenol in 30% sodium hydroxide solution at 80–85° (81%). There is substitution of the bromine atom by the benzoyl group.
Yield:1137-42-4 99 %Chromat.
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);potassium carbonate in toluene at 120; for 0.0833333 h;Microwave irradiation;Suzuki coupling;
Steps:
General procedure for the microwave-assisted reactions
General procedure: In a typical experiment, a tube equipped with a magnetic stirrer was charged with 1.0 mmol of base, 0.5 mmol of boronic acid, 0.005 mmol of Pd2dba3 or 1.0% weight of the supported palladium catalyst, 1.0 mL of the solvent and 0.5 mmol of the aroyl chloride. This tube was sealed and the content was subjected to focused microwave irradiation at 250 W for 7.0 min with 120 °C as the general final temperature. Then the reaction mixture was cooled to room temperature and diluted with 8.0 mL of ethyl ether, filtered and the residue was washed with two portions of ethyl ether (8.0 mL). This solution was analyzed by GC/MS. For the isolation of the ketones, after cooling to room temperature, the reaction mixture was diluted with ethyl acetate and washed with aqueous NaOH 10% and water, filtered over celite, was treated the same manner described for the representative procedure under MW heating and analyzed by GC/MS.
References:
De Luna Martins, Daniela;Aguiar, Lúcia C.S.;Antunes, Octavio A.C. [Journal of Organometallic Chemistry,2011,vol. 696,# 15-16,p. 2845 - 2849] Location in patent:experimental part
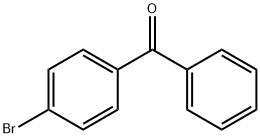
90-90-4
240 suppliers
$10.00/5g

1137-42-4
517 suppliers
$6.00/25g
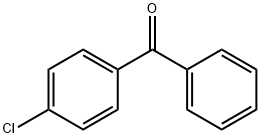
134-85-0
634 suppliers
$5.00/25g

1137-42-4
517 suppliers
$6.00/25g
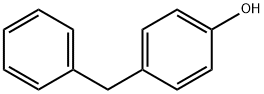
101-53-1
123 suppliers
$8.00/1g

1137-42-4
517 suppliers
$6.00/25g