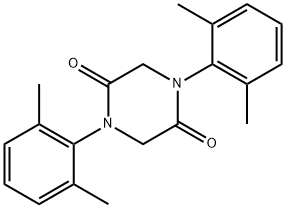
Lidocaine Impurity synthesis
- Product Name:Lidocaine Impurity
- CAS Number:70336-49-1
- Molecular formula:C20H22N2O2
- Molecular Weight:322.4

1131-01-7
374 suppliers
$7.00/5g

70336-49-1
25 suppliers
inquiry
Yield:-
Reaction Conditions:
in 1,2-dimethoxyethane;mineral oil;
Steps:
1 Example 1
Example 1 -- Preparation of 1,4-di-(2,6-dimethylphenyl)-2,5-piperazinedione A round-bottom flask was purged with nitrogen and filled with161 g (0.81 mol) 2,6-dimethyl-alpha-chloroacetanilide and 700 ml dimethoxyethane. The flask was immersed in an ice bath. 32.9 g (0.81 mol) of sodium hydride (50% dispersion in mineral oil) was then added in small portions to the flask over a 1-hour period. The ice bath was removed and the reaction mixture was stirred at about 25° C. for 16 hours. The reaction mixture was filtered to give the product as white crystals. The mother liquor was evaporated under reduced pressure to give additional white crystalline product. The solid products were washed with water, combined, washed with 200 ml ethyl ether, and dried in a vacuum oven. The total yield of product, m.p. 233-235° C., was 109 g (83.4%). Elemental analysis for C20 H22 N2 O2 showed:
References:
US4140791,1979,A