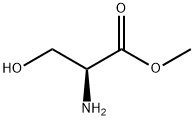
L-SERINE METHYL ESTER synthesis
- Product Name:L-SERINE METHYL ESTER
- CAS Number:2788-84-3
- Molecular formula:C4H9NO3
- Molecular Weight:119.12

67-56-1
776 suppliers
$7.29/5ml-f
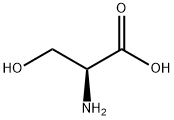
56-45-1
780 suppliers
$5.00/25g

2788-84-3
29 suppliers
$367.00/0.25G
Yield:2788-84-3 98%
Reaction Conditions:
with thionyl chloride
Steps:
Following the synthetic route depicted below the L-amino acids were treated with methanol and sulfoxide chloride to convert into the corresponding L-amino acid methyl esters II (98% yield) in the present invention. The methyl esters IIa-d were acylated by use of benzoyl chloride and N-benzoyl-L-amino acid methyl esters of IIIa-d were obtained (81% yield). Using sodium borohydride as the reduction agent the benzoylated methyl esters were reduced to N-benzoylaminoglycols IVa-d successfully (97% yield).
References:
Zen, Hayley;Peng, Shiqi;Bi, Lanrong;Zhao, Ming;Wang, Chao US2005/43396, 2005, A1 Location in patent:Page/Page column 3
1676-81-9
193 suppliers
$15.00/1g

2788-84-3
29 suppliers
$367.00/0.25G
5680-80-8
457 suppliers
$10.00/5g

2788-84-3
29 suppliers
$367.00/0.25G
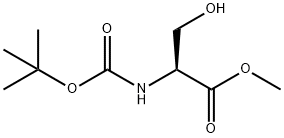
2766-43-0
308 suppliers
$10.00/1g

2788-84-3
29 suppliers
$367.00/0.25G

56-45-1
780 suppliers
$5.00/25g

2788-84-3
29 suppliers
$367.00/0.25G