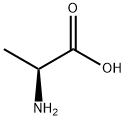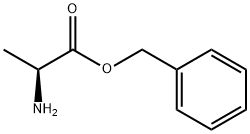
L-ALANINE BENZYL ESTER synthesis
- Product Name:L-ALANINE BENZYL ESTER
- CAS Number:17831-01-5
- Molecular formula:C10H13NO2
- Molecular Weight:179.22
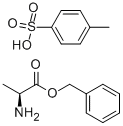
42854-62-6
297 suppliers
$7.00/1g

17831-01-5
14 suppliers
inquiry
Yield: 92%
Reaction Conditions:
with triethylamine in water;toluene at 5; for 2.5 h;
Steps:
1
Into a 500 mL separable flask equipped with a thermometer and a stirring apparatus was charged 33.0 g of p-toluenesulfonic acid salt of L-alanine benzyl ester (content analysis value according to high performance liquid chromatography internal standard method: L-alanine benzyl ester 47.7 wt% (87.9 mmol), p-toluenesulfonic acid 50.8 wt%) and 99.0 g of water, these were mixed, then, a nitrogen atmosphere is made in the flask. To the mixture was added 94.5 g of toluene, then, the mixture was cooled to 5 C, and 10.8 g (107 mmol) of triethylamine was dropped over a period of 2 hours while keeping the same temperature. The resultant mixture was stirred at 5 C for 30 minutes, and allowed to stand still, then, subjected to a liquid separation treatment (organic layer obtained in this stage is referred to as organic layer A). 94.5 g of toluene was charged while keeping the resultant aqueous layer at 5 C, the mixture was stirred at the same temperature for 30 minutes and allowed to stand still, then, subjected to a liquid separation treatment (organic layer obtained in this stage is referred to as organic layer B). The organic layer B was subjected to a concentration treatment under a reduced pressure of 8.0 to 4.7 kPa at an inner temperature of 31 to 42 C, then, the concentrate of the resultant organic layer B, and the organic layer A were mixed, and the mixture was subjected to a concentration treatment under a reduced pressure of 9.3 to 4.0 kPa at an inner temperature of 35 to 42 C, to obtain 60.0 g of a toluene solution containing L-alanine benzyl ester. The solution was analyzed by a high performance liquid chromatography internal standard method to find inclusion of 14.5 g (80.7 mmol) of L-alanine benzyl ester. The yield of L-alanine benzyl ester against p-toluenesulfonic acid salt was 92%.
References:
Sumitomo Chemical Company, Limited EP2078710, 2009, A1 Location in patent:Page/Page column 11
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

17831-01-5
14 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

17831-01-5
14 suppliers
inquiry
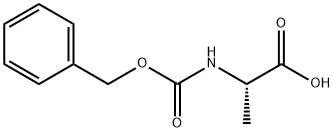
1142-20-7
459 suppliers
$5.00/1g

17831-01-5
14 suppliers
inquiry