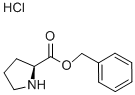
L-Proline benzyl ester hydrochloride synthesis
- Product Name:L-Proline benzyl ester hydrochloride
- CAS Number:16652-71-4
- Molecular formula:C12H16ClNO2
- Molecular Weight:241.71
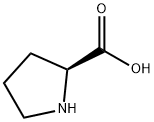
147-85-3
1034 suppliers
$6.00/25g
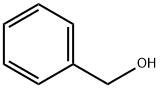
100-51-6
1429 suppliers
$5.00/100g

16652-71-4
332 suppliers
$6.00/5g
Yield:16652-71-4 93%
Reaction Conditions:
Stage #1:benzyl alcohol with thionyl chloride at 0;Inert atmosphere;
Stage #2:L-proline at 0 - 20; for 50 h;Inert atmosphere;
Steps:
2.4 (S)-2-((Benzyloxy)carbonyl)pyrrolidin-1-ium chloride (8)
Benzyl alcohol (70 mL, 651 mmol) was cooled to 0 °C under nitrogen and 7.0 mL thionyl chloride (91.2 mmol) was added. l-Proline (5.0 g, 43.4 mmol) was then added and the mixture was stirred at 0 °C under nitrogen for 2 h.
The mixture was warmed to room temperature and stirring continued for 48 h.
The reaction mixture was then poured into 300 mL diethyl ether and stored at -20 °C for 7 days.
The precipitate formed was collected by filtration, washed with diethyl ether, and dried under vacuum to give 8 as white solid (9.88 g, 93% yield); mp 142.1-144.0 °C; lit: 143-144 °C. 1H NMR (300 MHz, CDCl3): δ 7.41-7.21 (m, 5H), 5.16 (s, 2H), 3.80 (dd, J = 3.83, 5.9 Hz, 1H), 3.15-3.01 (m, 1H), 3.00-2.82 (m, 1H), 2.42-2.21 (m, 1H), 2.13 (dd, J = 12.9 Hz, 7.5 Hz, 1H), 1.92-1.62 (m, 3H).
13C NMR (75 MHz, CDCl3): δ 175.5, 136.0, 128.8, 128.5, 128.3, 66.9, 59.9, 47.2, 30.4, 25.6. Anal. Calcd for C12H16ClNO2: C, 59.63; H, 6.67; N, 5.79. Found: C, 59.50; H, 6.86; N, 5.64.
25
References:
Li, Zhiliang;Lebedyeva, Iryna O.;Golubovskaya, Vita M.;Cance, William G.;Alamry, Khalid A.;Faidallah, Hassan M.;Dennis Hall;Katritzky, Alan R. [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 15,p. 5056 - 5060]
37787-77-2
0 suppliers
inquiry

16652-71-4
332 suppliers
$6.00/5g

100-51-6
1429 suppliers
$5.00/100g

16652-71-4
332 suppliers
$6.00/5g
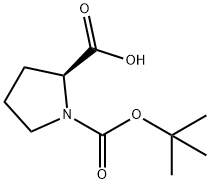
15761-39-4
615 suppliers
$5.00/10g

16652-71-4
332 suppliers
$6.00/5g