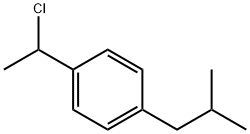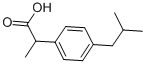
Ibuprofen synthesis
- Product Name:Ibuprofen
- CAS Number:15687-27-1
- Molecular formula:C13H18O2
- Molecular Weight:206.28
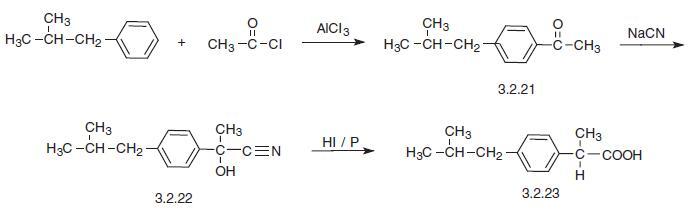
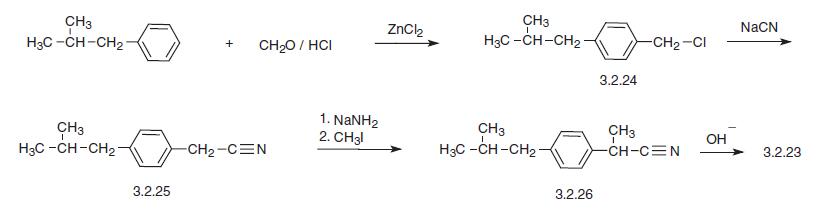
Another way to synthesize ibuprofen consists of the chloromethylation of iso-butylbenzene, giving 4-iso-butylbenzylchloride (3.2.24). This product is reacted with sodium cyanide, making 4-iso-butylbenzyl cyanide (3.2.25), which is alkylated in the presence of sodium amide by methyl iodide into 2-(4-iso-butylbenzyl)propionitrile (3.2.26). Hydrolysis of the resulting product in the presence of a base produces ibuprofen (3.2.23).
Yield:15687-27-1 99%
Reaction Conditions:
with hydrogenchloride;water;triphenylphosphine;1% Rh/C in butanone at 115; under 41372.9 Torr;
Steps:
145 EXAMPLE 145
[01224] A 50 ml stirred autoclave was charged with the following reactants [01225] 1-(4'-isobutylphenyl)ethyl chloride: 2.5 g [01226] 1% Rh/C: 0.1 g [01227] triphenyl phosphine: 0.08165 g [01228] 50% HCl: 1.2 ml [01229] Methyl ethyl ketone: 21.5 ml. [01230] The contents of the autoclave were flushed with nitrogen and then many times with carbon monoxide. Thereafter, the contents were heated to 115° C. After the temperature is attained, the autoclave was pressurised to 800 psig with carbon monoxide, stirring started and it was observed that gas absorption commenced immediately. For synthesis of ibuprofen, the pressure in the autoclave was maintained constant using carbon monoxide and the progress of the reaction was monitored by observing the pressure drop and by liquid sampling. The reaction was continued until the pressure drop was too low. The reactor was then cooled and the liquid phase analyzed by gas chromatography. [01231] The GC analysis showed TOF of 330 h-1 and 98% conversion of 1-(4'-isobutylphenyl)ethyl chloride with ibuprofen selectivity of 99%. The catalyst was filtered out and the solvent evaporated and the reaction mixture re-dissolved in toluene. The filtrate was treated with saturated solution of sodium bicarbonate two-three times and the aqueous phase separated to obtain the sodium salt of ibuprofen, which on hydrolysis with acid and extraction with dichloromethane, evaporation and vacuum distillation gives the pure product.
References:
US6660883,2003,B1 Location in patent:Page column 71-72
36039-36-8
114 suppliers
$63.00/100mg

15687-27-1
847 suppliers
$5.00/1g
41283-72-1
67 suppliers
$39.00/250mg

15687-27-1
847 suppliers
$5.00/1g
201230-82-2
1 suppliers
inquiry
40150-92-3
74 suppliers
$23.00/100mg

15687-27-1
847 suppliers
$5.00/1g