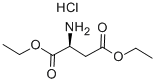
H-ASP(OET)-OET HCL synthesis
- Product Name:H-ASP(OET)-OET HCL
- CAS Number:16115-68-7
- Molecular formula:C8H16ClNO4
- Molecular Weight:225.67
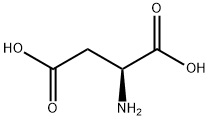
56-84-8
914 suppliers
$5.00/25g

16115-68-7
158 suppliers
$10.00/5 g
Yield:-
Reaction Conditions:
in ethanol;benzene
Steps:
10 L-Aspartic acid, diethyl ester, hydrochloride
EXAMPLE 10 L-Aspartic acid, diethyl ester, hydrochloride Hydrogen chloride was bubbled into a stirred suspension of L-aspartic acid (133 g.; 1.00 mole) in absolute ethanol (1.95 l.) at room temperature for 2 hours. The resulting solution was heated at reflux for 5 hours, cooled to room temperature, then evaporated in vacuo. The residue was dissolved in benzene (500 ml.). The solution was heated to reflux, and the water present was removed by means of a Dean-Stark trap. The solution was then concentrated at reduced pressure to an oil which slowly crystallized on standing. The solid was suspended in ether (1.3 l.), collected on a filter, washed with ether (800 ml.), then dried; yield of L-aspartic acid, diethyl ester, hydrochloride, 216 g. (95.7%); m.p., 99°-103°. This material was recrystallized from 1.25 l. of acetone-ether (4:1) to give 164.6 g.(76.2% recovery) of product suitable for further transformation; m.p., 106.5°-107.5°.
References:
Starks Associates, Inc. US4215070, 1980, A

64-17-5
757 suppliers
$10.00/50g

56-84-8
914 suppliers
$5.00/25g

16115-68-7
158 suppliers
$10.00/5 g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

16115-68-7
158 suppliers
$10.00/5 g