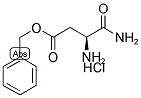
H-ASP(OBZL)-NH2 HCL synthesis
- Product Name:H-ASP(OBZL)-NH2 HCL
- CAS Number:199118-68-8
- Molecular formula:C11H15ClN2O3
- Molecular Weight:258.7

199118-68-8
43 suppliers
$45.00/100mg
![1,2-Pyrrolidinedicarboxylic acid, 1-[(4-bromophenyl)methyl] ester, (S)- (9CI)](/CAS/20200515/GIF/144332-75-2.gif)
144332-75-2
4 suppliers
inquiry
![L-α-Asparagine, 1-[[(4-bromophenyl)methoxy]carbonyl]-L-prolyl-, phenylmethyl ester](/CAS/20210305/GIF/1618080-03-7.gif)
1618080-03-7
0 suppliers
inquiry
Yield:1618080-03-7 3.88 g
Reaction Conditions:
with 4-methyl-morpholine;O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate in N,N-dimethyl-formamide at 20; for 24 h;Inert atmosphere;
Steps:
To a solution of3c (4.34 g, 12.7 mmol) in THF (120mL) was added MeOH (12 mL) and a solution of LiOH (1.83 g, 76.1 mmol) in water(12 mL). The reaction was stirred at room temperature for 3 h. The reaction wasquenched upon addition of 2 N HCl (200 mL) and extracted with EtOAc (2 x 200mL). The combined organic extracts were dried over Na2SO4and concentrated under vacuum. The residue was dissolved in DMF (120 mL), addedH-Asp(OBzl)-CONH2?HCl (3.27 g, 12.7 mmol), HBTU (5.70 g, 15.0 mmol)and NMM (1.97 mL, 14.5 mmol). The reaction was stirred at room temperature for20 h. The reaction was diluted with water (500 mL) and stirred for 5 mins.Isolated the white ppt formed by vacuum filtration to afford intermediate diamide(3.88 g, 58%); 1H-NMR (400 MHz, d6-DMSO)δ 8.27 (t, J = 8.0 Hz, 1H), 7.57 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.41-7.29 (m, 6H), 7.25(d, J = 8.0 Hz, 1H), 7.19 (br s, 1H),7.07 (br s, 1H), 5.15-4.92 (m, 4H), 4.63-4.56 (m, 1H), 4.18 (ddd, J = 24.0, 8.0, 1.5 Hz, 1H), 3.51-3.35(m, 2H), 2.86 (dd, J = 16.0, 8.0 Hz,1H), 2.66 (dd, J = 12.0, 8.0 Hz, 1H),2.19-2.02 (m, 1H), 1.89-1.70 (m, 3H); LC-MS (210/254 nM) >98%, m/z 488.09/490.11 [M-CONH2].The intermediate amide (3.85 g, 7.24 mmol) was dissolved in DMF (120 mL), addedcyanuric chloride (1.34 g, 7.24 mmol) and stirred at room temperature for 3 h.The reaction was diluted with water (450 mL) and extracted with EtOAc (2 x 300mL). The combined organic extracts were washed with water (2 x 450 mL) andbrine (500 mL), dried over Na2SO4 and concentrated undervacuum to afford 4c (3.71 g, 99%) as a clear yellow oil; 1H-NMR (400MHz, CDCl3) δ 7.61 (br s, 1H), 7.40 (d, J = 8.0 Hz, 2H), 7.29 (s, 5H), 7.14 (d, J = 8.0 Hz, 2H), 5.16-5.08 (m, 3H), 5.00 (q, J = 12.0 Hz, 2H), 4.24-4.17 (m, 1H), 3.46-3.28 (m, 2H), 2.86-2.75(m, 2H), 2.17-2.06 (m, 1H), 1.96-1.82 (m, 3H); LC-MS (210/254 nM) >98%, m/z 514.08/516.10 [M+H].
References:
Higgins, Catherine;Bouazzaoui, Samira;Gaddale, Kishore;D'Costa, Zenobia;Templeman, Amy;O'Rourke, Martin;Young, Andrew;Scott, Christopher;Harrison, Tim;Mullan, Paul;Williams, Rich [Bioorganic and Medicinal Chemistry Letters,2014,vol. 24,# 11,p. 2521 - 2524] Location in patent:supporting information