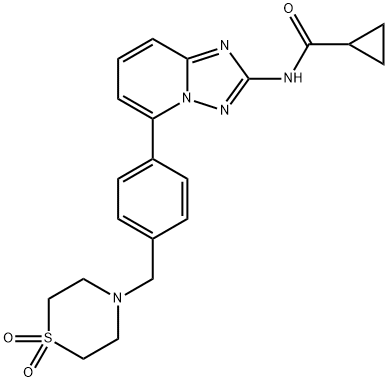
Filgotinib synthesis
- Product Name:Filgotinib
- CAS Number:1206161-97-8
- Molecular formula:C21H23N5O3S
- Molecular Weight:425.5
With cyclopropanyl chloride in an amidated reaction in a system consisting of N-methylmorpholine and 1,4-dioxane,The amidation reaction time was 4 h,The amidation reaction temperature was 50 ° C,The molar ratio of the intermediate (III), cyclopropanyl chloride, N-methylmorpholine and 1,4-dioxane was 1: 2.8: 2.5:TLC plate to determine the reaction is completed, cooled to room temperature,Adding methylene chloride and water, separating the organic phase with water,Then washed with brine, dried over magnesium sulfate,Evaporated to dryness and the residue was purified over a silica gel column [elution solvent: ethyl acetate / n-hexane (3: 7 v / v)To obtain a solid yellowish solid,That is, Filgotinib, yield 91.2%.
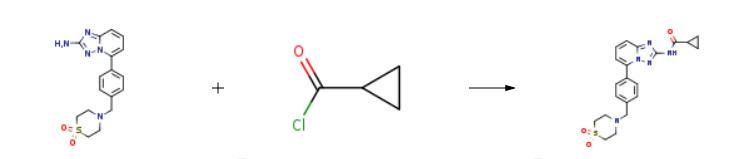
![[1,2,4]Triazolo[1,5-a]pyridin-2-amine, 5-[4-[(1,1-dioxido-4-thiomorpholinyl)methyl]phenyl]-](/CAS/20200611/GIF/1257705-09-1.gif)
1257705-09-1
30 suppliers
inquiry
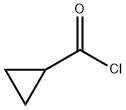
4023-34-1
370 suppliers
$12.00/5g

1206161-97-8
242 suppliers
$25.00/1mg
Yield:1206161-97-8 91.2%
Reaction Conditions:
with 4-methyl-morpholine in 1,4-dioxane at 50; for 4 h;Green chemistry;Reagent/catalyst;Solvent;Temperature;
Steps:
3.F F) Preparation of Filgotinib:
The intermediate (III) obtained in step E)With cyclopropanyl chloride in an amidated reaction in a system consisting of N-methylmorpholine and 1,4-dioxane,The amidation reaction time was 4 h,The amidation reaction temperature was 50 ° C,The molar ratio of the intermediate (III), cyclopropanyl chloride, N-methylmorpholine and 1,4-dioxane was 1: 2.8: 2.5:TLC plate to determine the reaction is completed, cooled to room temperature,Adding methylene chloride and water, separating the organic phase with water,Then washed with brine, dried over magnesium sulfate,Evaporated to dryness and the residue was purified over a silica gel column [elution solvent: ethyl acetate / n-hexane (3: 7 v / v)To obtain a solid yellowish solid,That is, Filgotinib, yield 91.2%The reaction of this step is the same as in Example 1.
References:
Suzhou Fushilai Pharmaceutical Co., Ltd.;Mo Guoning CN104987333, 2017, B Location in patent:Paragraph 0039; 0040; 0047; 0054; 0061
39093-93-1
239 suppliers
$5.00/250mg
![N-(5-(4-(bromomethyl)phenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-yl)cyclopropanecarboxamide](/CAS/20180703/GIF/1206163-59-8.gif)
1206163-59-8
6 suppliers
inquiry

1206161-97-8
242 suppliers
$25.00/1mg
![4-[4-(4,4,5,5-tetramethyl[1,3,2]dioxaborolan-2-yl)benzyl]thiomorpholine 1,1-dioxide](/CAS/20180629/GIF/1092563-25-1.gif)
1092563-25-1
68 suppliers
$65.00/10mg
![cyclopropanecarboxylic acid (5-bromo-[1,2,4]triazolo[1,5-a]pyridin-2-yl)-amide](/CAS/20150408/GIF/1142943-96-1.gif)
1142943-96-1
106 suppliers
inquiry

1206161-97-8
242 suppliers
$25.00/1mg
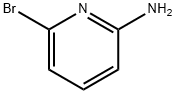
19798-81-3
452 suppliers
$5.00/1g

1206161-97-8
242 suppliers
$25.00/1mg
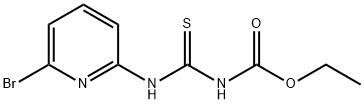
1010120-59-8
23 suppliers
$195.00/1 G

1206161-97-8
242 suppliers
$25.00/1mg