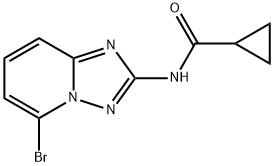
cyclopropanecarboxylic acid (5-bromo-[1,2,4]triazolo[1,5-a]pyridin-2-yl)-amide synthesis
- Product Name:cyclopropanecarboxylic acid (5-bromo-[1,2,4]triazolo[1,5-a]pyridin-2-yl)-amide
- CAS Number:1142943-96-1
- Molecular formula:C10H9BrN4O
- Molecular Weight:281.11
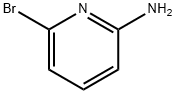
![5-Bromo-[1,2,4]triazolo[1,5-a]pyridin-2-ylamine](/CAS2/GIF/1010120-55-4.gif)
1010120-55-4
166 suppliers
$6.00/100mg
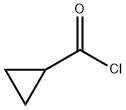
4023-34-1
366 suppliers
$12.00/5g
![cyclopropanecarboxylic acid (5-bromo-[1,2,4]triazolo[1,5-a]pyridin-2-yl)-amide](/CAS/20150408/GIF/1142943-96-1.gif)
1142943-96-1
107 suppliers
inquiry
Yield: 85.8%
Reaction Conditions:
Stage #1:5-Bromo-[1,2,4]Triazolo[1,5-a]pyridine-2-ylamine with triethylamine in dichloromethane at -20 - 0; for 0.5 h;
Stage #2:cyclopropanecarboxylic acid chloride in dichloromethane at 20;
Stage #3: with ammonium hydroxide in methanol at 20; for 1 h;
Steps:
1.3 Step 3 Synthesis of N- (5-bromo- [1,2,4] triazolo [1,5-a] pyridin-2-yl) cyclopropylacetamide (V)
Below 0 ° C, 16.0 g (0.075 mol) of intermediate IV was added to 80 mL of dichloromethane and stirred until completely dissolved. Control the temperature at -20 ~ 0 , after slowly adding 26mL (0.18mol) triethylamine, continue stirring for 0.5h, dropwise add 17mL (0.18mol) cyclopropyl formyl chloride in methylene chloride (20mL) solution to the reaction solution After dropping, react at room temperature for 2-5h. After the reaction was completed, the reaction solution was washed with water (70 mL × 2) and 70 mL of saturated saline in this order, and the organic layer was evaporated to dryness under reduced pressure. The crude product was added to 80 mL of methanol, 34 mL of ammonia water was added, and the reaction was performed at room temperature for 1 h. After the reaction was completed, the reaction solution was concentrated under reduced pressure, 150 mL of water was added to the reaction solution, the aqueous layer was extracted twice with dichloromethane (200 mL × 2), the organic layers were combined, and washed sequentially with water (100 mL × 2) and saturated 100 mL brine. The organic layer was dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was evaporated to dryness under reduced pressure, and dried to give 48.1 g of light yellow solid with a yield of 85.8%;
References:
Shenzhen Kunjian Chuangxin Pharmaceutical Institute;Jiang Yuyang;Lu Kuan;Zhang Cunlong;Wu Weibin;Chen Dawei CN111072655, 2020, A Location in patent:Paragraph 0046; 0059; 0064-0065
19798-81-3
444 suppliers
$5.00/1g
![cyclopropanecarboxylic acid (5-bromo-[1,2,4]triazolo[1,5-a]pyridin-2-yl)-amide](/CAS/20150408/GIF/1142943-96-1.gif)
1142943-96-1
107 suppliers
inquiry
1010120-59-8
22 suppliers
$195.00/1 G
![cyclopropanecarboxylic acid (5-bromo-[1,2,4]triazolo[1,5-a]pyridin-2-yl)-amide](/CAS/20150408/GIF/1142943-96-1.gif)
1142943-96-1
107 suppliers
inquiry
![5-Bromo-[1,2,4]triazolo[1,5-a]pyridin-2-ylamine](/CAS2/GIF/1010120-55-4.gif)
1010120-55-4
166 suppliers
$6.00/100mg
![cyclopropanecarboxylic acid (5-bromo-[1,2,4]triazolo[1,5-a]pyridin-2-yl)-amide](/CAS/20150408/GIF/1142943-96-1.gif)
1142943-96-1
107 suppliers
inquiry