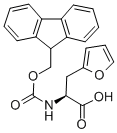
FMOC-L-2-FURYLALANINE synthesis
- Product Name:FMOC-L-2-FURYLALANINE
- CAS Number:159611-02-6
- Molecular formula:C22H19NO5
- Molecular Weight:377.39
146725-85-1
15 suppliers
inquiry
28920-43-6
528 suppliers
$5.00/5g

159611-02-6
120 suppliers
$22.00/100mg
Yield: 1.3 g (53%)
Reaction Conditions:
with LiOH;sodium hydrogencarbonate in 1,4-dioxane
Steps:
6.c c
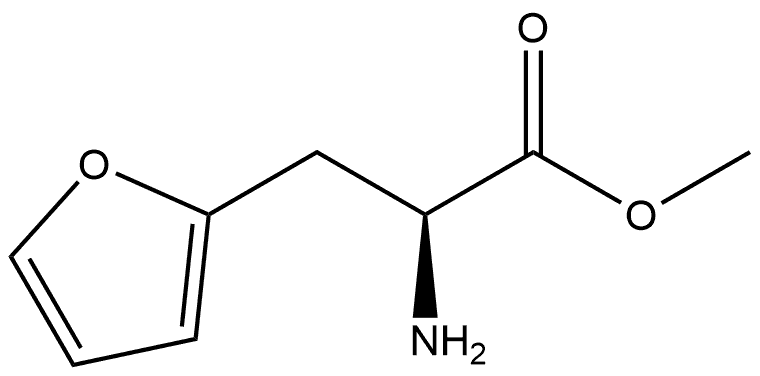
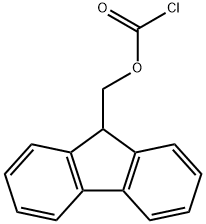
c (S)-2-(9-Fluorenylmethyloxycarbonylamino)-3-(2-furyl)-propionic acid Methyl (S)-2-amino-3-(2-furyl)propionate (1 g, 6.5 mmol) was mixed with 2M LiOH (3.27 ml, 6.5 mmol) and dioxane (3.27 ml) at 0° C. and stirred overnight under N2. The next day a TLC control (CHCl3 /MeOH/NH3 aq 1/1/0.1) of the reaction mixture showed no ester present. 1M NaHCO3 (9.75 ml, 9.75 mmol) and 9-fluorenylmethyloxycarbonyl chloride (2.5 g, 9.75 mmol) dissolved in dioxane (10 ml) were added to the above solution and the stirring continued for a further 1 hour. Dioxane was removed under reduced pressure and the aqueous solution acidified with a 10% KHSO4 solution to pH 2. The solution was extracted with CHCl3 (3*20 ml), dried (MgSO4), evaporated and purified by flash chromatography (Hexane/ethyl acetate/acetic acid 10/10/1). Yield: 1.3 g (53%), white crystals. 1 H NMR (CDCl3): δ2.95 (dd, 1H, J 9.9, J 15.2 Hz), 3.08 (dd, 1H, J 4.4, J 15.2 Hz), 3.6 (br.s, 1H), 4.16-4.26 (m, 4H), 6.12 (d, 1H, J 2.9 Hz), 6.33 (t, 1H, J 2.3 Hz), 7.29 (t, 2H, J 7.32 Hz), 7.39 (t, 2H, J 7.48 Hz), 7.49 (br.s, 1H), 7.64 (dd, 2H, J 7.17, J 2.14 Hz), 7.85 (d, 2H, J 7.48 Hz). 13 C NMR (CDCl3): δ29.8, 46.98, 66.04, 107.45, 110.85, 120.48, 125.57, 125.63, 127.50, 128.07, 141.06, 142.21, 144.09, 151.84, 156.26, 160.35.
References:
Nycomed Imaging AS US5629293, 1997, A