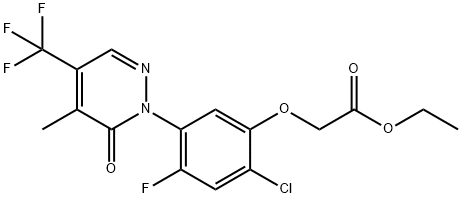
FLUFENPYR-ETHYL synthesis
- Product Name:FLUFENPYR-ETHYL
- CAS Number:188489-07-8
- Molecular formula:C16H13ClF4N2O4
- Molecular Weight:408.73
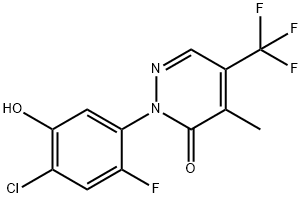
188489-05-6
0 suppliers
inquiry
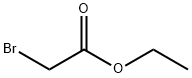
105-36-2
355 suppliers
$5.00/5 g

188489-07-8
26 suppliers
$502.50/5MG
Yield:-
Reaction Conditions:
in diethyl ether;water;N,N-dimethyl-formamide
Steps:
P.1 Production Example 1
Then, 3.2 g (9.9 mmol) of 2-[2-fluoro-4-chloro-5-hydroxyphenyl]-4-methyl-5-trifluoromethylpyridazin-3-one was dissolved in about 50 ml of N,N-dimethyl-formamide, to which 0.44 g (11 mmol) of sodium hydride (60 wt % oil dispersion) was added at room temperature. The mixture was left stand at room temperature for 30 minutes and then cooled with ice, to which 1.8 g (11 mmol) of ethyl bromoacetate was added. The mixture was stirred at room temperature for 1 hour, to which diethyl ether and water were added in this order to make an extraction. The organic layer was washed with 10% aqueous hydrochloric acid solution, saturated aqueous sodium hydrogencarbonate solution, and saturated aqueous sodium chloride solution in this order, and then dried over anhydrous magnesium sulfate. The solvent was distilled out under reduced pressure, and the residue was subjected to silica gel column chromato-graphy, which afforded 2.4 g (5.5 mmol) of ethyl 2-chloro-4-fluoro-5-(4-methyl-5-trifluoromethyl-3-pyridazinon-2-yl)phenoxyacetate (compound [1] wherein R is ethyl; hereinafter referred to as compound A), m.p., 102.0° C.
References:
Sumitomo Chemical Company, Limited US6022829, 2000, A