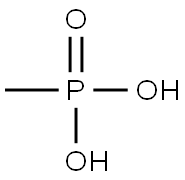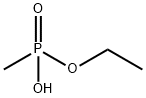
ETHYL METHYLPHOSPHONIC ACID synthesis
- Product Name:ETHYL METHYLPHOSPHONIC ACID
- CAS Number:1832-53-7
- Molecular formula:C3H9O3P
- Molecular Weight:124.08
591-22-0
313 suppliers
$11.19/25ML
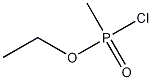
5284-09-3
1 suppliers
inquiry
53123-88-9
777 suppliers
$9.00/10mg

1832-53-7
47 suppliers
$96.90/1g
Yield:-
Reaction Conditions:
with sodium hydrogencarbonate in dichloromethane;ethyl acetate
Steps:
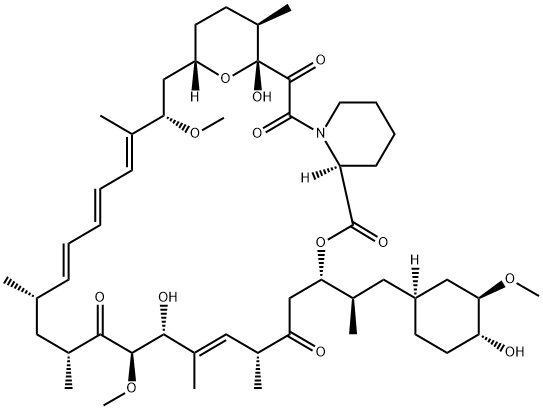
1 Methyl-phosphonic acid ethyl ester C-43 rapamycin ester
Methyl-phosphonic acid ethyl ester C-43 rapamycin ester To a cooled (0° C.) solution of rapamycin (0.1 g, 0.109 mmol) in 1.5 mL of dichloromethane was added a solution of 3,5-lutidine (0.088 g, 0.82 mmol) in 0.25 mL of dichloromethane, under an atmosphere of N2, followed immediately by a solution of ethyl methylphosphonochloridate (0.078 g, 0.547 mmol) in 0.25 mL of dichloromethane. The colorless reaction solution was stirred at 0° C. for 3 h (reaction monitored by MS; reaction sample diluted directly with 50:50CH3CN/H2O, 1 drop DMSO, prior to analysis). The cold (0° C.) reaction solution was diluted with ~20 mL EtOAc then transferred to a separatory funnel containing EtOAc (150 mL) and saturated NaHCO3 (100 mL). Upon removing the aqueous layer, the organic layer was washed successively with ice cold 1N HCl (1*100 mL), saturated NaHCO3 (1*100 mL), and brine (1*100 mL), then dried over MgSO4 and concentrated. The crude product was purified by silica gel flash chromatography (eluted with 0.5:10:3:3 MeOH/DCM/EtOAc/hexane) to provide 0.024 g of a white solid (~2:1 diastereomeric mixture): 1H NMR (300 MHz, CDCl3) d 4.19 (m, 1 Ha, 1 Hb), 4.15-4.01 (m, 3 Ha, 3 Hb), 1.56-1.27 (m, 6 Ha, 6 Hb); 31P NMR (121 MHz, CDCl3) d 32.1, 29.9; 1043 m/z (M+Na).
References:
Berstein, David L.;Metcalf III, Chester A.;Rozamus, Leonard W.;Wang, Yihan US2003/220297, 2003, A1
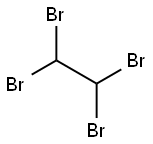
79-27-6
212 suppliers
$13.00/25g
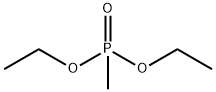
683-08-9
124 suppliers
$29.00/1g
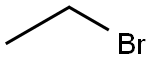
74-96-4
442 suppliers
$9.00/5g
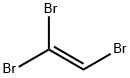
598-16-3
37 suppliers
$64.00/25g

1832-53-7
47 suppliers
$96.90/1g