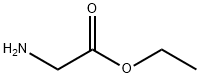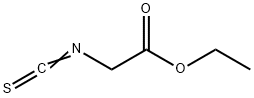
ETHYL ISOTHIOCYANATOACETATE synthesis
- Product Name:ETHYL ISOTHIOCYANATOACETATE
- CAS Number:24066-82-8
- Molecular formula:C5H7NO2S
- Molecular Weight:145.18

75-15-0
262 suppliers
$40.00/100 g
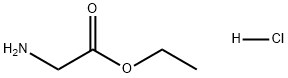
623-33-6
652 suppliers
$5.00/10g

24066-82-8
143 suppliers
$31.00/1g
Yield: 48%
Reaction Conditions:
Stage #1:carbon disulfide;glycine ethyl ester hydrochloride with 4-methyl-morpholine in dichloromethane at 20; for 0.166667 h;
Stage #2: with 4‐(4,6‐dimethoxy‐1,3,5‐triazin‐2‐yl)‐4‐methylmorpholinium toluene‐4‐sulfonate in dichloromethane at 20; for 0.5 h;
Steps:
3.2.4. General Procedure for Compounds 9a-j
General procedure: Hydrochloride 8a-j (2 mmol, 1 equiv.), NMM (0.66 mL, 6 mmol, 3 equiv. or 1.32 mL,12 mmol, 6 equiv. for 8i) and CS2 (0.36 mL, 6 mmol, 3 equiv. or 0.72 mL, 12 mmol, 6 equiv.for 8i) were dissolved in dry DCM (5 mL) in 10 mL round bottom flask equipped witha magnetic bar, and stirred 10 min at rt. Next, DMT/NMM/TsO- (1) (0.828 g, 2 mmol,1 equiv. or 1.656 g, 4 mmol, 2 equiv. for 8i) was added. The reaction was mixed 30 min atrt. After that the reaction mixture was diluted with DCM (50 mL) and washed by H2O(5 mL), 1 N HCl (2 x 5 mL), H2O (5 mL), and dried under anhydrous MgSO4. The crudeproducts were purified by flash chromatography on silica gel (7-8 g) using mixture hexane:EtOAc 20: 1 as eluent. Pure isothiocyanates 9a-j were isolated after careful evaporation ofthe solvent and removal of volatile residues under reduced pressure.
References:
Janczewski, ?ukasz;Kolesińska, Beata;Kr?giel, Dorota [Molecules,2021,vol. 26,# 9]
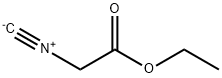
2999-46-4
222 suppliers
$13.43/1gm:

24066-82-8
143 suppliers
$31.00/1g
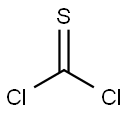
463-71-8
152 suppliers
$29.89/5g

623-33-6
652 suppliers
$5.00/10g

24066-82-8
143 suppliers
$31.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

24066-82-8
143 suppliers
$31.00/1g