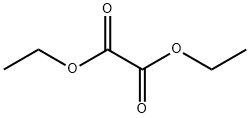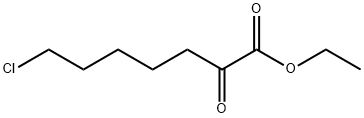
Ethyl 7-chloro-2-oxoheptanoate synthesis
- Product Name:Ethyl 7-chloro-2-oxoheptanoate
- CAS Number:78834-75-0
- Molecular formula:C9H15ClO3
- Molecular Weight:206.67
Yield:78834-75-0 99.1%
Reaction Conditions:
Stage #1: 1-bromo-5-chloropentane;oxalic acid diethyl ester in tetrahydrofuran at -25 - -10; for 1 h;Inert atmosphere;
Stage #2: with hydrogenchloride in tetrahydrofuran;water at 0;Inert atmosphere;Reagent/catalyst;
Steps:
1-10 A method for synthesizing ethyl 7-chloro-2-oxoheptanoate, characterized in that the method comprises the following steps:
Step 1. Under dry N2 protection, add 7.3 g of diethyl oxalate, 9.3 g of 1-bromo-5-chloropentane and a four-necked flask equipped with a condenser, a thermometer, a stirrer and a 50 ml constant pressure dropping funnel. a mixture of 30 ml of tetrahydrofuran;Step 2, at -25 ° C,Quickly add 1.0g PSIM-MgBr,During the dropwise addition reaction, the reaction temperature is kept at about -10 ° C, and after the completion of the dropwise addition, the reaction is continued for 1 h;Step 3. Hydrolyze with 15 ml of 4 M hydrochloric acid, and ensure the reaction temperature is below 0 °C during acid hydrolysis. The organic layer was separated, and the aqueous layer was extracted with dichloromethane, and washed with a saturated sodium chloride solution, and shaken with a NaHS03 saturated solution;Step 4: After the shaking is completed, the organic layer and the aqueous layer are neutralized with a saturated NaHC03 solution, and the organic layer is rotated to evaporate the tetrahydrofuran. The organic layer is combined, neutralized with saturated NaHC03, washed with NaHS03, then washed with NaCl until neutral, and evaporated.Evaporation under reduced pressure gave ethyl 7-chloro-2-oxoheptanoate.
References:
CN108752206,2018,A Location in patent:Paragraph 0008; 0010-0018; 0029
![2-Heptenoic acid, 7-chloro-2-[[[(1S)-2,2-dimethylcyclopropyl]carbonyl]amino]-, ethyl ester, (2E)-](/CAS/20210305/GIF/1022895-93-7.gif)
1022895-93-7
1 suppliers
inquiry

78834-75-0
236 suppliers
$6.00/1g
75885-58-4
272 suppliers
$5.00/1g
52387-36-7
55 suppliers
inquiry

78834-75-0
236 suppliers
$6.00/1g

2009-83-8
379 suppliers
$7.00/25g

78834-75-0
236 suppliers
$6.00/1g
1174680-06-8
0 suppliers
inquiry

78834-75-0
236 suppliers
$6.00/1g