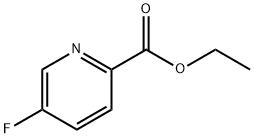
ETHYL 5-FLUOROPYRIDINE-2-CARBOXYLATE synthesis
- Product Name:ETHYL 5-FLUOROPYRIDINE-2-CARBOXYLATE
- CAS Number:148541-70-2
- Molecular formula:C8H8FNO2
- Molecular Weight:169.15

41404-58-4
405 suppliers
$5.00/1g

64-17-5
728 suppliers
$10.00/50g
201230-82-2
1 suppliers
inquiry

148541-70-2
33 suppliers
$116.64/250mgs:
Yield: 78%
Reaction Conditions:
with 1,1'-bis-(diphenylphosphino)ferrocene;triethylamine;palladium diacetate in N,N-dimethyl-formamide at 50; under 760.051 Torr; for 3 h;
Steps:
A6.a
The mixture of 2-bromo-5-fluoropyridine (1.00 g, 0.5.68 mmol), palladium(II) acetate (127 mg, 0.568 mmol), l,l'-bis(diphenylphosphino)ferrocene (630 mg, 1.14 mmol) and triethylamine (1.6 mmol) in ethanol/N,N-dimethylformamide (1:1, 30 ml) was stirred under carbon monoxide atmosphere (1 atm) at 50 °C for 3 hours. H2O (20 ml) was added and the whole was filtrated through a pad of Celite. The filtrate was extracted with ethyl acetate, washed with H2O and brine, dried (MgSO4) and evaporated under reduced pressure. The residue was purified by column chromatography on silica (ethyl acetate/hexane, 20/80-50/50) to give ethyl 5- fluoropicolinate (754 mg, 78 %, colorless solid).
References:
SHIONOGI & CO., LTD.;EURO-CELTIQUE S.A WO2008/8398, 2008, A2 Location in patent:Page/Page column 354
107504-08-5
268 suppliers
$7.00/250mg

64-17-5
728 suppliers
$10.00/50g

148541-70-2
33 suppliers
$116.64/250mgs: