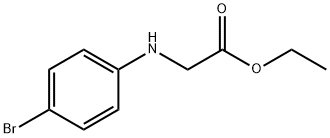
ETHYL [(4-BROMOPHENYL)AMINO]ACETATE synthesis
- Product Name:ETHYL [(4-BROMOPHENYL)AMINO]ACETATE
- CAS Number:2521-92-8
- Molecular formula:C10H12BrNO2
- Molecular Weight:258.11
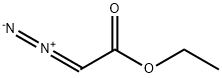
623-73-4
149 suppliers
$27.30/25ML
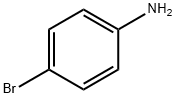
106-40-1
468 suppliers
$12.00/5g
![ETHYL [(4-BROMOPHENYL)AMINO]ACETATE](/StructureFile/ChemBookStructure3/GIF/CB3718609.gif)
2521-92-8
16 suppliers
$45.00/100mg
Yield:2521-92-8 97%
Reaction Conditions:
NCPS-Ru in toluene at 70;Conversion of starting material;
Steps:
8
Example 8NCPS-Ru Catalyzed Intermolecular N-H Insertion of AminesThe invention further relates to intermolecular N-H insertion of amine with ethyl diazoacetate catalyzed by NCPS-Ru. Ethyl diazoacetate (1.0 mmol) was added in one portion to a mixture of amine (1.1 mmol) and NCPS-Ru (1.0 mol %) in toluene (2 mL) at 70° C. After the addition, stirring was continued until all of the diazo compounds had been consumed. Upon addition of hexane (2 mL) the reaction mixture was centrifuged, and aliquots were taken from the supernatant liquid for product identification and quantitation by 1H NMR spectroscopy. To obtain a pure product, the supernatant was separated and evaporated to dryness by rotary evaporation, and the residue was loaded onto a silica gel for column chromatography.The carbenoid insertion into N-H bonds is an attractive carbenoid transformation for the synthesis of α-amino carboxylic compounds.[16] In this work, we found that the NCPS-Ru catalyzed intermolecular N-H insertion reactions could be performed without using a slow addition procedure or an inert atmosphere. The N-H insertion products were obtained in high yields by one pot reaction of amine and ethyl diazoacetate in toluene at 70° C. in open atmosphere (i.e. without Ar/N2 protection). Complete substrate conversion was observed within 1 h (Table 8). No diazo compound coupling product was formed.In the literature, most reported metal-catalyzed intermolecular N-H insertion reactions using diazo compounds were conducted in a millimole scale.[16] In this work, we have examined the feasibility of scaling up the intermolecular N-H insertion reaction of aniline and ethyl diazoacetate using 0.1 mole of substrate. (Table 8, entry 8). Ethyl diazoacetate (0.10 mol) was added in one portion to a mixture of aniline (0.11 mol) and NCPS-Ru (0.1 mol %) in toluene at 70° C. in open atmosphere. Complete substrate conversion was formed within 1 h. N-phenylglycine ethyl ester was obtained in 97% yield. At a lower catalyst loading (0.01 mol %) intermolecular N-H insertion in a 0.1 mole scale took a longer reaction time (4 days) for complete consumption of ethyl diazoacetate, no diazo coupling products (fumarte/maleate) were detected by 1H NMR analysis of the reaction mixture. N-phenylglycine ethyl ester was obtained in 93% yield.; TABLE 8 NCPS-Ru 1 catalyzed intermolecular N-H insertion of amines[a] Entry Substrate ProductYield [%][b] 1 99 2 97 3 97 4 83 5 91 6 89 7 60 8 97 [a]A EDA (1.0 mmol) was added in one portion to a mixture of amine (1.1 mmol) and NCPS-Ru 1 (1.0 mol %) in toluene at 70° C. in open atmosphere.[b]Isolated yield.[c]A EDA (0.10 mol) was added in one portion to a mixture of aniline (0.11 mol) and NCPS-Ru 1 (0.1 mol %) in toluene at 70° C. in open atmosphere.
References:
US2011/9617,2011,A1 Location in patent:Page/Page column 4; 9
105-39-5
404 suppliers
$10.00/5g

106-40-1
468 suppliers
$12.00/5g
![ETHYL [(4-BROMOPHENYL)AMINO]ACETATE](/StructureFile/ChemBookStructure3/GIF/CB3718609.gif)
2521-92-8
16 suppliers
$45.00/100mg
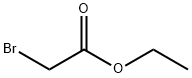
105-36-2
351 suppliers
$5.00/5G

106-40-1
468 suppliers
$12.00/5g
![ETHYL [(4-BROMOPHENYL)AMINO]ACETATE](/StructureFile/ChemBookStructure3/GIF/CB3718609.gif)
2521-92-8
16 suppliers
$45.00/100mg
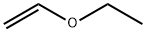
109-92-2
272 suppliers
$17.00/25mL

106-40-1
468 suppliers
$12.00/5g
![ETHYL [(4-BROMOPHENYL)AMINO]ACETATE](/StructureFile/ChemBookStructure3/GIF/CB3718609.gif)
2521-92-8
16 suppliers
$45.00/100mg

106-40-1
468 suppliers
$12.00/5g
![ETHYL [(4-BROMOPHENYL)AMINO]ACETATE](/StructureFile/ChemBookStructure3/GIF/CB3718609.gif)
2521-92-8
16 suppliers
$45.00/100mg