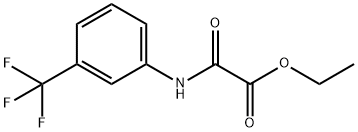
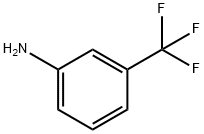
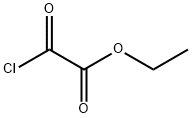
Yield:17738-86-2 100%
Reaction Conditions:
with triethylamine in ethyl acetate at 0 - 19; for 2.5 h;Product distribution / selectivity;
Steps:
1a
Ethyl oxo { [3 -(trifluoromethyl)phenyl] amino } acetate 3-Trifluoromethylaniline (350 g, 1 eq), triethylamine (351 g, 1.6 eq) and ethyl acetate (4.55 L) were charged to a flask, placed under a nitrogen atmosphere and cooled to 0 0C. Ethyl oxalyl chloride (356 g, 1.2 eq) was added dropwise keeping the reaction temperature between 5-10 0C. The reaction mixture was warmed to 16 0C and held at 16-19 0C for 2.5 hours. The reaction was then quenched with water (2.33 L). The aqueous layer was separated and extracted with ethyl acetate (1.05 L). The organic layers were combined and washed with 2M HCl (0.88 L); water (0.88 L); saturated aqueous sodium bicarbonate (0.58 L); and water (0.88 L). The organic layer was concentrated to dryness to yield the title compound (568.2 g, 100%) as an orange/yellow solid.1H NMR (300 MHz, DMSOd6) δ 11.09 (s, IH), 8.19 (s, IH), 8.03 (d, J = 8.0 Hz, IH),7.61 (t, J = 8.1 Hz, IH), 7.51 (d, J = 7.8 Hz, IH), 4.32 (q, J = 7.5 Hz, 2H), 1.32 (t, / =7.0 Hz, 3H). APCI-MS m/z: 262.0 [MH+].
References:
WO2009/61271,2009,A1 Location in patent:Page/Page column 40-41