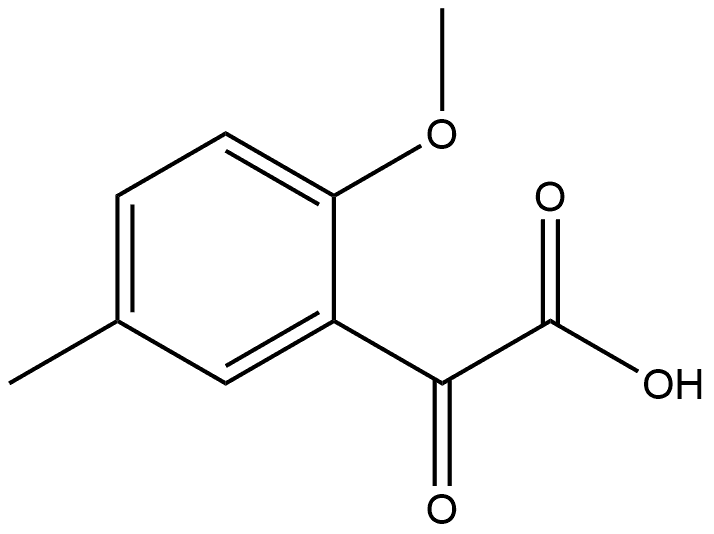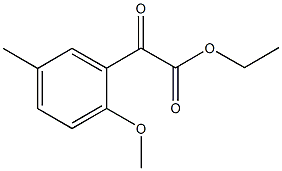
ETHYL 2-METHOXY-5-METHYLBENZOYLFORMATE synthesis
- Product Name:ETHYL 2-METHOXY-5-METHYLBENZOYLFORMATE
- CAS Number:859775-82-9
- Molecular formula:C12H14O4
- Molecular Weight:222.24
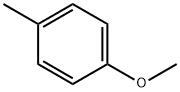
104-93-8
400 suppliers
$6.00/25g
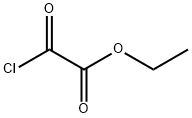
4755-77-5
353 suppliers
$9.00/25g

859775-82-9
14 suppliers
$252.00/1g
Yield:859775-82-9 98%
Reaction Conditions:
Stage #1: 1-methoxy-4-methylbenzenewith Aluminum Chloride in dichloromethane at 0; for 0.166667 h;
Stage #2: oxalyl chloride monoethyl ester in dichloromethane at 0 - 20; for 16 h;
Steps:
10.A ethyl 2-(2-methoxy-5-methylphenyl)-2-oxoacetate
Example 10A
ethyl 2-(2-methoxy-5-methylphenyl)-2-oxoacetate
1-Methoxy-4-methylbenzene (6.30 mL, 50 mmol, Aldrich) was added to a suspension of aluminum chloride (8.00 g, 60.0 mmol) in dichloromethane (100 mL) at 0° C.
After stirring for 10 minutes, ethyl 2-chloro-2-oxoacetate (6.70 mL, 60.0 mmol, Aldrich) was added dropwise, and the reaction was allowed to slowly warm to ambient temperature.
After 16 hours, the reaction was quenched with 1 M hydrochloric acid (200 mL) and vigorously stirred for 15 minutes.
The layers were then separated and the organic phase was washed with saturated NaHCO3 and brine, dried with MgSO4, filtered through a pad of diatomaceous earth, and concentrated under reduced pressure to afford the title compound (10.9 g, 49.0 mmol, 98% yield).
1H NMR (400 MHz, CDCl3) δ ppm 7.67 (d, J=2.4 Hz, 1H), 7.38 (dd, J=8.4, 2.4 Hz, 1H), 6.88 (d, J=8.4 Hz, 1H), 4.38 (q, J=7.1 Hz, 2H), 3.84 (s, 3H), 2.32 (s, 3H), 1.39 (t, J=7.1 Hz, 3H). MS(APCI+) m/z 223.6 (M+H)+.
References:
US2022/213041,2022,A1 Location in patent:Paragraph 0396
117373-23-6
0 suppliers
inquiry

859775-82-9
14 suppliers
$252.00/1g