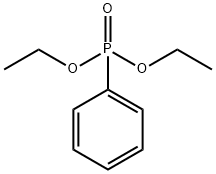
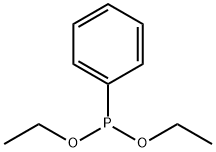
Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate synthesis
- Product Name:Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
- CAS Number:84434-11-7
- Molecular formula:C18H21O3P
- Molecular Weight:316.33
The reaction mixture was diluted with 100 mL water, neutralized with saturated sodium bicarbonate, and the organic layer was separated and washed twice with water. Excess oxidant was reduced with aqueous sodium sulfite, and the organic layer was separated and washed again. The mixture was dehydrated with anhydrous sodium sulfate, filtered to remove the desiccant.
To the dehydrated organic layer, 0.11 mol sodium hydroxide was added, followed by dropwise addition of 0.55 mol diethyl sulfate at 60-80°C. After the reaction was complete, the mixture was adjusted to pH 8-10 with sodium hydroxide, washed with water, and the organic layer was separated. The organic layer was dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure to yield Ethyl phenyl(2,4,6-trimethylbenzoyl)phosphinate.
Yield:84434-11-7 328 kg
Reaction Conditions:
with triethylamine at 75; for 2 h;Large scale;
Steps:
4 Arylphosphine oxide section
The ethyl diphenylphosphonite is continuously input into the high-level dripping tank under negative pressure,In a temperature zone around 75 degrees Celsius, dropwise add to the acylphosphine oxygen synthesis kettle pre-mixed with 230 kg of 2,4,6-trimethylbenzoyl chloride and 8 kg of triethylamine.The reaction is completed in about 2 hours, and the chloroethane gas generated during the process is input into the condensation recovery device under the protection of static electricity.26 kg of dilute hydrochloric acid was added to the reaction solution to remove residual acid chloride, 1000 liters of toluene were pumped in, and the reaction was stirred to obtain a homogeneous liquid.Put the reaction liquid into the washing tank from the bottom valve of the synthesis tank,The temperature is controlled at about 50 degrees Celsius, and the reaction solution is washed with alkaline water, acid water, and neutral water in sequence.The organic phase is automatically input into the vacuum desolventizing kettle, the temperature is controlled not to exceed 75 degrees Celsius, and the toluene solvent is recovered.After adding 700 liters of ethanol to the residue, it was heated with activated carbon to decolorize, and the reaction solution was filtered while it was hot.The ethanol is removed to obtain 328 kg of TPO-L light yellow liquid product (purity above 96%).
References:
CN112778360,2021,A Location in patent:Paragraph 0075-0076
487-68-3
343 suppliers
$6.00/5g

64-17-5
756 suppliers
$10.00/50g
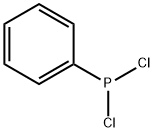
644-97-3
0 suppliers
$14.00/5g

84434-11-7
330 suppliers
inquiry
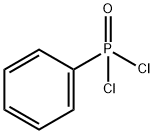
824-72-6
219 suppliers
$15.00/25g
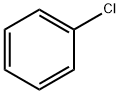
108-90-7
671 suppliers
$10.00/25G
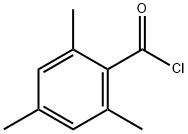
938-18-1
309 suppliers
$9.00/25g

84434-11-7
330 suppliers
inquiry
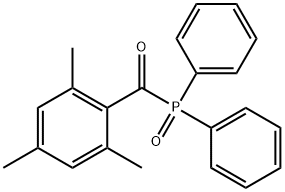
75980-60-8
485 suppliers
$14.00/5g

644-97-3
0 suppliers
$14.00/5g

84434-11-7
330 suppliers
inquiry