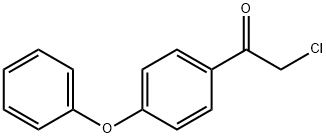
Ethanone,2-chloro-1-(4-phenoxyphenyl)- synthesis
- Product Name:Ethanone,2-chloro-1-(4-phenoxyphenyl)-
- CAS Number:13075-63-3
- Molecular formula:C14H11ClO2
- Molecular Weight:246.69
Yield:2251-54-9 64%
Reaction Conditions:
with aluminum (III) chloride in dichloromethane at -5 - 20;
Steps:
4 Preparation of 2-chloro-1- (4- (phenoxy) phenyl) ethanone (Compound I-5-1-1)
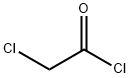
Add 20g to the three-necked flaskDiphenoxy ether and 100 ml of dichloromethane,Add 23.5g anhydrous A1C13,Plus drying tube, reflux condenser and exhaust gas absorption device, ice salt bath cooling to 0 , slowly dropping chloroacetyl chloride,Control the temperature at -5 ~ 5 , about 20min drop finished, remove the ice salt water bath, naturally returned to room temperature,Continue to stir the reaction 2 ~ 3h, TLC monitoring the basic reaction is complete.The reaction solution was slowly poured into 10% ice in dilute hydrochloric acid, pH = 4, stirred for 30 minutes,100 ml of dichloromethane, the organic phases were combined, washed with 3 x 100 ml of water,2% NaHCO3 solution adjusted to neutral after the liquid, 100ml washed 1, anhydrous MgSO4 dry, filtered,Concentrated under reduced pressure and recrystallized to give an off-white solid, i.e., Compound I-5-1-1, yield: 64%.
References:
CN107129458,2017,A Location in patent:Paragraph 0144; 0145; 0146